Chapter 9 Atrioventricular Conduction Abnormalities
General Considerations
Anatomy and Physiology of the Atrioventricular Junction
Internodal and Intraatrial Conduction
Evidence indicates the presence of preferential impulse propagation from the sinus node to the atrioventricular node (AVN)—that is, higher conduction velocity between the nodes in some parts of the atrium than in other parts. However, whether preferential internodal conduction is caused by fiber orientation, size, or geometry or by the presence of specialized preferentially conducting pathways located between the nodes has been controversial.1–3
Atrioventricular Node
The AVN is an interatrial structure, measuring approximately 5 mm long, 5 mm wide, and 0.8 mm thick in adults. The AVN is located beneath the RA endocardium at the apex of the triangle of Koch. The triangle of Koch is septal and constitutes the RA endocardial surface of the muscular AV septum. It is bordered anteriorly by the annulus of the septal leaflet of the tricuspid valve, posteriorly by the tendon of Todaro, and inferiorly by the orifice of the CS ostium (CS os) (see Fig. 17-1). The central fibrous body is composed of a thickened area of fibrous continuity between the leaflets of the mitral and aortic valves, termed the right fibrous trigone, together with the membranous component of the cardiac septum. The tendon of Todaro runs within the eustachian ridge and inserts into the central fibrous body; the annulus of the septal leaflet of the tricuspid valve crosses the membranous septum.4–6
The compact AVN lies anterior to the CS os and directly above the insertion of the septal leaflet of the tricuspid valve, where the tendon of Todaro merges with the central fibrous body. Slightly more anteriorly and superiorly is where the His bundle (HB) penetrates the AV junction through the central fibrous body and the posterior aspect of the membranous AV septum.7 The compact node is adjacent to the central fibrous body on the right side but is uninsulated by fibrous tissue on its other sides, thus allowing contiguity with the atrial myocardium. Because the AV valves are not isoplanar (the attachment of the tricuspid valve into the most anterior part of the central body is a few millimeters apically relative to the mitral valve), the AVN lies just beneath the RA endocardium. When traced inferiorly, toward the base of the triangle of Koch, the compact AVN area separates into two extensions, usually with the artery supplying the AVN running between them. The prongs bifurcate toward the tricuspid and mitral annuli, respectively. The rightward posterior extensions have been implicated in the so-called slow pathway in AVN reentrant tachycardia (AVNRT) circuit.4–6
The normal AV junctional area can be divided into distinct regions: the transitional cell zone (which represents the approaches from the working atrial myocardium to the AVN), the compact AVN, and the penetrating part of the HB.8 The AVN and perinodal area are composed of at least three electrophysiologically distinct cells: the atrionodal (AN), nodal (N), and nodal-His (NH) cells. The AN region corresponds to the cells in the transitional region that are activated shortly after the atrial cells. Transitional cells are histologically distinct from both the cells of the compact AVN and the working atrial myocytes, and they are not insulated from the surrounding myocardium, but tend to be separated from one another by thin fibrous strands. Transitional cells do not represent conducting tracts but a bridge funneling atrial depolarization into the compact AVN via discrete AVN inputs (approaches). Transitional cells approaches connect the working atrial myocardium from the left and right sides of the atrial septum to the left and right margins of the compact node, with wider extensions inferiorly and posteriorly between the compact node and the CS os and into the eustachian ridge. In humans and animals, two such inputs are commonly recognized in the right septal region: the anterior (superior) approaches, which travel from the anterior limbus of the fossa ovalis and merge with the AVN closer to the apex of the triangle of Koch; and the posterior (inferior) approaches, which are located in the inferoseptal RA and serve as a bridge with the atrial myocardium at the CS os. Although both inputs have traditionally been assumed to be RA structures, growing evidence supports the AV conduction apparatus as a transseptal structure that reaches both atria. A third, middle group of transitional cells has also been identified to account for the nodal connections with the septum and LA.4–6
The N region corresponds to the region where the transitional cells merge with midnodal cells. The N cells represent the most typical of the nodal cells, which are smaller than atrial myocytes, are closely grouped, and frequently are arranged in an interweaving fashion. The N cells in the compact AVN appear to be responsible for the major part of AV conduction delay and exhibit decremental properties in response to premature stimulation because of their slow rising and longer action potentials. Fast pathway conduction through the AVN apparently bypasses many of the N cells by transitional cells, whereas slow pathway conduction traverses the entire compact AVN. Importantly, the recovery of excitability after conduction of an impulse is faster for the slow pathway than for the fast pathway, for reasons that are unclear.4
The NH region corresponds to the lower nodal cells, connecting to the insulated penetrating portion of the HB. Sodium (Na+) channel density is lower in the midnodal zone of the AVN than in the AN and NH cell zones, and the inward L-type calcium (Ca2+) current is the basis of the upstroke of the N cell action potential. Therefore, conduction is slower through the compact AVN than the AN and NH cell zones.9,10
The AVN is the only normal electrical connection between the atria and the ventricles; the fibrous skeleton acts as an insulator to prevent electrical impulses from entering the ventricles by any other route. The main function of the AVN is modulation of atrial impulse transmission to the ventricles, thereby coordinating atrial and ventricular contractions. The AVN receives, slows down, and conveys atrial impulses to the ventricles. A primary function of the AVN is to limit the number of impulses conducted from the atria to the ventricles. This function is particularly important during fast atrial rates (e.g., during atrial fibrillation [AF] or atrial flutter), in which only a few impulses are conducted to the ventricles, and the remaining impulses are blocked in the AVN. Additionally, fibers in the lower part of the AVN can exhibit automatic impulse formation, and the AVN may serve as a subsidiary pacemaker.5,6,9
The AVN region is innervated by a rich supply of cholinergic and adrenergic fibers. Sympathetic stimulation shortens AVN conduction time and refractoriness, whereas vagal stimulation prolongs AVN conduction time and refractoriness. The negative dromotropic response of the AVN to vagal stimulation is mediated by activation of the inwardly rectifying potassium (K+) current IKACh, which results in hyperpolarization and action potential shortening of AVN cells, increased threshold of excitation, depression of action potential amplitude, and prolonged conduction time. The positive dromotropic effect of sympathetic stimulation arises as a consequence of activation of the L-type Ca2+ current.7
His Bundle
The HB connects with the distal part of the compact AVN and passes through the fibrous core of the central fibrous body in a leftward direction (away from the RA endocardium and toward the ventricular septum). The HB then continues through the annulus fibrosis (where it is called the nonbranching portion) as it penetrates the membranous septum, along the crest of the left side of the interventricular septum, for 1 to 2 cm and then divides into the right and left bundle branches.4
Proximal cells of the penetrating portion are heterogeneous and resemble those of the compact AVN; distal cells are larger, similar to cells in the proximal bundle branches and ventricular myocytes. The HB is insulated from the atrial myocardium by the membranous septum and from the ventricular myocardium by connective tissue of the central fibrous body, thus preventing atrial impulses from bypassing the AVN. The area of fibrous continuity between the aortic and mitral valves adjacent to the membranous septum marks the HB as viewed from the left ventricle (LV). Viewed from the aorta, the HB passes beneath the part of the membranous septum that adjoins the interleaflet fibrous triangle between the right and the noncoronary sinuses.4
Pathophysiology of Atrioventricular Block
Congenital Atrioventricular Block
Congenital complete AV block is thought to result from embryonic maldevelopment of the AVN (and, much less frequently, the His-Purkinje system [HPS]), mainly secondary to a lack of connection between the atria and the peripheral conduction system, with fatty replacement of the AVN and nodal approaches.4 The incidence of congenital complete AV block varies from 1 in 15,000 to 1 in 22,000 live births. The defect usually occurs proximal to the HB, and QRS duration is shorter than 120 milliseconds.11 Maternal lupus, caused by antibodies targeting intracellular ribonucleoproteins that cross the placenta to affect the fetal heart but not the maternal heart, is responsible for 60% to 90% of cases of congenital complete AV block.12
Approximately 50% of patients with congenital AV block have concurrent congenital heart disease (e.g., congenitally corrected transposition of the great vessels, AV discordance, ventricular septal defects, AV canal defect, tricuspid atresia, and Ebstein anomaly of the tricuspid valve).13 The AV conduction system may be displaced if atrial and ventricular septa are malaligned, AV arrangements are discordant, or the heart is univentricular. Generally, if the AV conduction system is displaced, it will also tend to be more fragile and susceptible to degeneration, thus placing patients at greater risk for AV block.14
Hereditary Progressive Cardiac Conduction Disease
Cardiac ion channelopathies have been described as a rare cause of familial forms of AV block. Mutations in the SCN5A gene (encoding the alpha-subunit of the cardiac Na+ channel) and the KCNJ2 gene (encoding the inward rectifier Kir2.1, a critical component of the cardiac inward K+ rectifier current, IK1) have been associated with AV block. Additionally, mutations in the PRKAG2 gene (encoding the gamma2 regulatory subunit of adenosine monophosphate–activated protein kinase) have been described in patients with Wolff-Parkinson-White syndrome and AV conduction block.15,16
Acquired Atrioventricular Block
Drugs
Various drugs can impair conduction and cause AV block. Digoxin and beta blockers act indirectly on the AVN through their effects on the autonomic nervous system. Calcium channel blockers and other antiarrhythmic drugs, such as amiodarone and dronedarone, act directly to slow conduction in the AVN. Class I and III antiarrhythmic drugs can also affect conduction in the HPS that results in infranodal block. These effects, however, typically occur in patients with preexisting conduction abnormalities. Patients with a normal conduction system function rarely develop complete heart block as a result of using antiarrhythmic agents.17
Acute Myocardial Infarction
AV block occurs in 12% to 25% of all patients with acute myocardial infarction (MI); first-degree AV block occurs in 2% to 12% second-degree AV block occurs in 3% to 10%, and third-degree AV block occurs in 3% to 7%. First-degree AV block and type 1 second-degree (Wenckebach) AV block occur more commonly in inferior MI, usually caused by increased vagal tone and generally associated with other signs of vagotonia, such as sinus bradycardia and responsiveness to atropine and catecholamine stimulation. Wenckebach AV block in the setting of acute inferior MI is usually transient (resolving within 48 to 72 hours of MI) and asymptomatic, and it rarely progresses to high-grade or complete AV block. Wenckebach AV block occurring later in the course of acute inferior MI is less responsive to atropine and probably is associated with ischemia of the AVN or the release of adenosine during acute MI. In this setting, Wenckebach AV block rarely progresses to more advanced block and commonly resolves within 2 to 3 days of onset. The site of conduction block is usually in the AVN.
Complete AV block occurs in 8% to 13% of patients with acute MI. It can occur with anterior or inferior acute MI. In the setting of acute inferior MI, the site of the block is usually at the level of the AVN, and it results in a junctional escape rhythm with a narrow QRS complex and a rate of 40 to 60 beats/min. The block tends to be reversed by vagolytic drugs or catecholamines and usually resolves within several days. Development of complete AV block in the setting of acute anterior MI, however, is associated with a higher risk of ventricular tachycardia (VT) and ventricular fibrillation, hypotension, pulmonary edema, and in-hospital mortality. In this setting, the block is usually associated with ischemia or infarction of the HB or bundle branches and is less likely to be reversible. Complete AV block during acute anterior MI is often preceded by bundle branch block (BBB), fascicular block, or type 2 second-degree AV block. The escape rhythm usually originates from the bundle branch and Purkinje system, with a rate less than 40 beats/min and a wide QRS complex. In general, patients who develop transient or irreversible AV block are older and have a larger area of damage associated with their acute MI.11,18
Degenerative Diseases
Progressive cardiac conduction disease (including Lev disease or Lenègre disease) manifests as progressive slowing of electrical conduction through the atria, AVN, HB, Purkinje fibers, and ventricles, accompanied by an age-related degenerative process, in which fibrosis affects only the cardiac conduction system. Complete AV block can develop and cause syncope or sudden death. Lev disease is a result of proximal bundle branch calcification or fibrosis and is often described as senile degeneration of the conduction system. It is postulated as a hastening of the aging process by hypertension and arteriosclerosis of the blood vessels supplying the conduction system. Lenègre disease is a sclerodegenerative process that occurs in a younger population and involves the more distal portions of the bundle branches. As noted, in heritable progressive cardiac conduction disease (referred to as hereditary Lenègre disease, progressive cardiac conduction disease, and familial AV block), conduction slowing may be attributed to loss-of-function mutations in SCN5A. Whether age-dependent fibrosis of the conduction system is a primary degenerative process in progressive cardiac conduction disease or a physiological process that is accelerated by Na+ current (INa) reduction is still unknown.16
Calcification of the aortic or (less commonly) mitral valve annulus can extend to the nearby conduction system and produce AV block. As noted, the HB penetrates the central fibrous body adjacent to the fibrous continuity between the aortic and mitral valves that is the usual site of dystrophic calcification, and extension of calcification can directly involve the HB or the origin of the left bundle branch, or both.4,11,18
Neuromyopathies
AV conduction disturbance is usually the major cardiac manifestation of neuromuscular diseases, including Becker muscular dystrophy, peroneal muscular dystrophy, Kearns-Sayre syndrome, Erb dystrophy, and myotonic muscular dystrophy. AV block can be an important cause of mortality in such cases.19
Infectious Diseases
Infective endocarditis (especially of the aortic valve) and myocarditis of various viral, bacterial, and parasitic causes (including Lyme disease, rheumatic fever, Chagas disease, tuberculosis, measles, and mumps) result in varying degrees of AV block. Complete AV block occurs in 3% of cases.17 Lyme carditis is of particular importance because in most cases, AV block resolves completely within weeks.
Iatrogenic
Cardiac surgery can be complicated by varying degrees of AV block caused by trauma and ischemic damage to the conduction system. AV block is most frequently associated with aortic valve replacement; less commonly, it occurs following coronary artery bypass grafting.20 Repair of congenital heart defects in the region of the conduction system, such as endocardial cushion malformations, ventricular septal defects, and tricuspid valve abnormalities, can lead to transient or persistent AV block.13,21,22 The block is usually temporary and is thought to be secondary to postoperative local inflammation. However, AV block can appear years later, usually in patients who had transient block just after the operation.23,24 Intracardiac catheter manipulation can inadvertently produce varying degrees of heart block, which is usually temporary. Alcohol septal ablation in patients with obstructive hypertrophic cardiomyopathy also can be complicated by AV block. Complete heart block can occur during right-sided heart catheterization in a patient with preexisting left BBB (LBBB) or during LV catheterization (LV angiography or ablation procedures) in a patient with preexisting right BBB (RBBB).25 AV block can also complicate catheter ablation of AVNRT, bypass tracts and atrial tachycardias in the AVN vicinity, as well as VTs originating in the interventricular septum adjacent to the HB.26
Vagally Mediated Atrioventricular Block
Vagally induced AV block can occur in otherwise normal patients, in those with cough or hiccups, and during swallowing or micturition when vagal discharge is enhanced.27 Vagally mediated AV block occurs in the AVN, is associated with a narrow QRS complex, and is generally benign. The block is characteristically paroxysmal and is often associated with clearly visible sinus slowing on the ECG because the vagal surge can cause simultaneous sinus slowing and AVN block. Additionally, transient AV block can occur secondary to enhanced vagal tone caused by carotid sinus massage, hypersensitive carotid sinus syndrome, or neurocardiogenic syncope. AV block in athletes is typically type 1 second-degree block, probably an expression of hypervagotonia related to physical training, and it resolves after physical deconditioning. This form of AV block may or may not be associated with sinus bradycardia because the relative effects of sympathetic and parasympathetic systems on the AVN and sinus node can differ.
Clinical Presentation
Symptoms in patients with AV conduction abnormalities are generally caused by bradycardia and loss of AV synchrony. Individuals with first-degree AV block are usually asymptomatic; however, patients with marked prolongation of the PR interval (longer than 300 milliseconds) can experience symptoms similar to those with pacemaker syndrome caused by loss of AV synchrony and atrial contraction against closed AV valves. Additionally, in patients with LV dysfunction, severe first-degree AV block can cause worsening of heart failure symptoms.28 Symptoms caused by more advanced AV block can range from exercise intolerance, easy fatigability, dyspnea on exertion, angina, mental status changes, dizziness, and near syncope to frank syncope.11,18 In patients with paroxysmal or intermittent complete heart block, symptoms are episodic, and routine ECGs may not be diagnostic.
Congenital AV block can be apparent in utero or at birth; however, many individuals have few or no symptoms and reach their teens or young adulthood before the diagnosis is made. Because of the presence of reliable subsidiary HB pacemakers with adequate rates (especially in the presence of catecholamines), syncope is rare with congenital complete AVN block. Some patients become symptomatic only when aging produces chronotropic incompetence of the HB rhythm.11,18
Natural History of Atrioventricular Block
The natural history of patients with AV block depends on the underlying cardiac condition; however, the site of the block and the resulting rhythm disturbances themselves contribute to the prognosis. Patients with first-degree AV block have an excellent prognosis, even when the condition is associated with chronic bifascicular block, because the rate of progression to third-degree AV block is low.28 Type 1 second-degree AV block is generally benign; however, when type 1 AV block occurs in association with bifascicular block, the risk of progression to complete heart block is significantly increased because of probable infranodal disease. Type 2 second-degree AV block, usually seen with BBB, carries a high risk of progression to advanced or complete AV block. The prognosis of 2:1 AV block depends on whether the site of block is within or below the AVN.11
The prognosis for patients with symptomatic acquired complete heart block is very poor in the absence of pacing, regardless of the extent of underlying heart disease. Once appropriate pacing therapy has been established, however, the prognosis depends on the underlying disease process.17 Complete heart block secondary to anterior MI carries a poor prognosis because of the coexisting extensive infarction and pump failure. In contrast, complete heart block secondary to idiopathic fibrosis of the conduction system in the absence of additional cardiac disease carries a more benign prognosis.11 AV block after valve surgery can recover; however, if conduction has not recovered by 48 hours after surgery, permanent pacing will likely be necessary.29
Diagnostic Evaluation of Atrioventricular Block
Autonomic Modulation
Whereas the AVN is richly innervated and highly responsive to both sympathetic and vagal stimuli, the HPS is influenced minimally by the autonomic nervous system. Carotid sinus massage increases vagal tone and worsens second-degree AVN block, whereas exercise and atropine improve AVN conduction because of sympathetic stimulation or parasympatholysis, or both. In contrast, carotid sinus massage can improve second-degree infranodal block by slowing the sinus rate and allowing HPS refractoriness to recover. Also, exercise and atropine worsen infranodal block because of the enhanced function of the sinus node and AVN and, as a consequence, the increased rate of impulses conducted to the HPS without changing HPS refractoriness.11,18
Electrocardiographic Features
First-Degree Atrioventricular Block (Delay)
Site of Block
The degree of PR interval prolongation and QRS duration can help predict the site of conduction delay. Very long (more than 300 milliseconds) or highly variable PR intervals suggest involvement of the AVN. Normal QRS duration also suggests involvement of the AVN.17,30
Atrioventricular Node
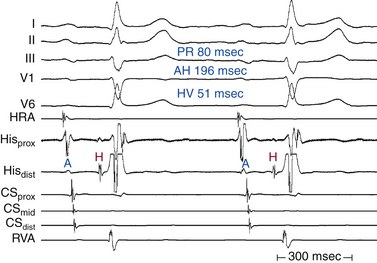
Although conduction delay can be anywhere along the AVN-HPS, the AVN is the most common site of delay (87% when the QRS complex is narrow, and more than 90% when the PR interval is longer than 300 milliseconds; Fig. 9-1).
His-Purkinje System
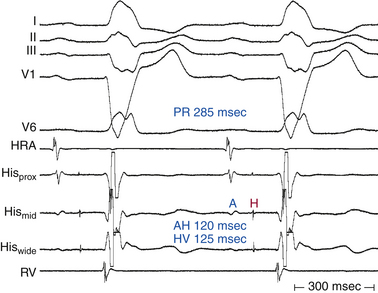
Intra-Hisian conduction delay or HPS disease can cause first-degree AV block. First-degree AV block in the presence of BBB is caused by infranodal conduction delay in 45% of cases. A combination of delay within the AVN and in the HPS must also be considered (Fig. 9-2).
Atrium
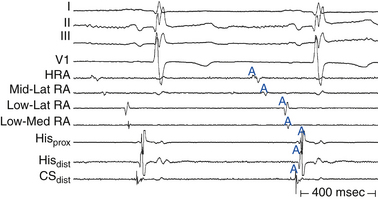
First-degree AV block caused by intraatrial or interatrial conduction delay is not uncommon. An LA enlargement pattern on the ECG (i.e., prolonged P wave duration) reflects the presence of interatrial conduction delay. RA enlargement can prolong the PR interval (Fig. 9-3). In certain cases of congenital structural heart disease, such as Ebstein anomaly of the tricuspid valve or endocardial cushion defects, intraatrial conduction delay can cause first-degree AV block.31
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree