Chapter 34 Assessment and orthotic management of gait dysfunction in individuals with traumatic brain injury
Most survivors of a brain insult have the potential for return of significant function and resumption of useful lives. The average age at which an injury occurs is bimodal, with a peak between 16 and 24 years and another later in life between 65 and 70 years. The incidence is higher in men. Life expectancy for patients who survive the first month after traumatic brain injury (TBI) is fairly long, particularly in the younger group. At least 70% of hemiplegic patients regain the ability to walk.3 Basic treatment principles are often waived on the erroneous assumption that the patient will not survive. For example, a patient whose fractures are left untreated because it is believed he or she will not survive poses a much greater treatment problem in later periods of recovery. Many patients will develop other complications due to injury to the central nervous system and polytrauma, such as spasticity, muscle overactivity, incoordination, and development of heterotopic ossification. Abnormal bone formation near proximal limb joints may take years to mature, and clinical experience dictates that surgical removal be delayed until maturity of the new bone has been reached.
Some features of normal gait control should be considered before discussing the different types of abnormal ambulation in patients with upper motor neuron. Normal gait involves a cyclic, highly automated, stereotypic movement pattern with rhythmic alternating motion of the trunk and extremities. In normal individuals, cycle-to-cycle variation of the details of the movement is relatively low.18 Locomotor action patterns are symmetrical and involve the entire body. In healthy subjects, gait is a skill that is mastered in a relatively uniform way. The three main functional goals of human ambulation are (1) to move from one place to another, (2) to move safely, and (3) to move efficiently. The gait of the patient after a TBI frequently is neither safe nor energy efficient.17 Compensatory movements necessary for ambulation produce abnormal or exaggerated vertical and horizontal displacements of the center of gravity. Impaired balance, sensory and visual deficits, and foot drag all can contribute to loss of balance, falls, and increased anxiety regarding ambulation and its accompanying risks.16 Cardiopulmonary fitness and joint range of motion may be impaired because of decreased intensity and frequency of exercise and ambulation, particularly early in the rehabilitation process.
Normal locomotion
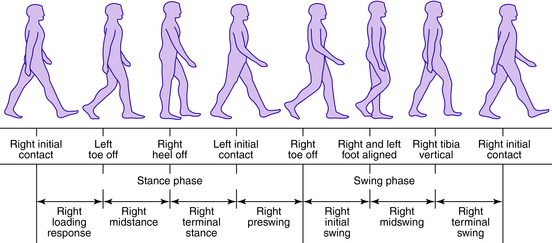
From the clinical standpoint, an understanding of the events occurring during the walking cycle is important so that pathologic locomotion can be correlated to cause and effect and precise timing during gait. Some basic terminology is reviewed here to help identify the components and events of the gait cycle.2,5,14 From the perspective of one limb, the gait cycle has two basic components: stance phase, during which the limb is in contact with the ground, and swing phase, during which the limb is off the ground. The stance phase can be subdivided into one event and four functional subphases: (1) initial contact, (2) loading response, (3) midstance, (4) terminal stance, and (5) preswing. The swing phase can be subdivided into three functional subphases: (1) initial swing, (2) midswing, and (3) terminal swing.2 A stride is one complete gait cycle and can be defined as the time from initial contact of one foot until the next initial contact of the same foot. Double support is the period of time during which both feet are in contact with the ground. Single support is the period when only one foot is in contact with the ground and is the equivalent to the swing period of the contralateral limb. Step length is the distance covered in the direction of progression during one step. The step period is the time measured from an event in one foot to the subsequent occurrence of the same event in the other foot (Fig. 34-1).
Temporal and spatial descriptive measures
In order to characterize gait, some basic output variables regarding temporospatial structure and sequencing of the stance and swing phases can be measured. These data can be obtained by measuring the distances and timing involved in the floor contacts of the feet. In the Gait and Motion Analysis Laboratory (GMAL), a device called the Gait Mat II is used to extract this type of information. A left and right “footprint record” is obtained, and a printout is generated that provides information about average and standard deviation of walking velocity, cadence, stance, and swing times for each side as well as stride lengths, step lengths, and base of support. Comparison of right and left parameters can be used to determine the extent of unilateral impairment or the effect of intervention.14
Kinematics
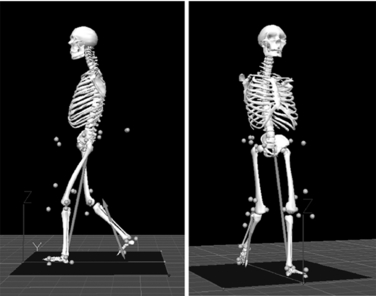
Kinematics provides a description of movements without regard to the forces generating them. The earlier techniques were photographic and cinematographic. Other techniques include the use of accelerometers and electrogoniometers. Modern systems use high-speed video/film recording with retro-reflective markers or, as in the GMAL, specialized optoelectronic apparatus in which active optical sources (e.g., infrared-emitting diodes) attached to the subject serve as markers. Time coincidence between controller illumination and camera reception uniquely identifies each light-emitting diode. Once the kinematic information is available as coordinate data, it can be processed and displayed as graphs or stick figures that demonstrate the gait sequence in the imaged plane (Fig. 34-2). Joint angles can be computed and displayed as a function of time or as a percentage of the stride period. Velocities and accelerations can be calculated. Video is a good alternative when instrumenting the subject is unacceptable.
Kinetics
Kinetic analysis deals with the forces, moments, and mechanical energies that develop during the course of walking. Ground reaction forces are generally measured using a triaxial force platform. Ideally, as in our laboratory, four platforms adjacent to one other should be used so that the forces transmitted through the contact surface for each foot can be recorded independently and simultaneously during sequential steps. The measured ground reaction forces are often normalized to body weight or expressed in Newtons. In the GMAL at MossRehab, the ground reaction force vector is visualized and superimposed in real time on the video image of the walking subject using laser optics. This is a useful tool that does not require patient instrumentation and facilitates determination of the effect of bracing or shoe modifications during walking.
Lower limb orthoses
Orthoses are devices applied to the external surface of the body part to achieve one or more of the following goals: (1) relieve pain, (2) immobilize musculoskeletal segments by limiting/directing joint motion, (3) reduce axial load, (4) prevent or correct deformity, and (5) improve function. Overall, orthoses can be divided into two major categories: corrective and accommodative devices. Corrective devices are meant to improve the position of the limb segment by stretching a contracture or correcting the alignment of skeletal structures.1 Accommodative devices are meant to provide additional support to already deformed joints and or tissues, to prevent further deformity, and ultimately to improve function. Orthoses can be further classified as static or dynamic. Dynamic orthoses permit movement of the involved joint(s) while controlling the direction or alignment of the movement and, at times, providing a substitute power source for weak muscles. The spectrum of orthotic devices available is broad, ranging from a simple plastic device applied across one joint to a much more complex device that is made of a variety of materials and crosses multiple joints.1
The orthotic components chosen depend on which functions they fulfill, but most orthoses consist of three basic elements: interface components, structural components, and joints. In orthoses of newer design, such as the plastic ankle–foot orthosis (AFO), differentiating the joints from the structural and interface components may be impossible.18
A complete orthotic prescription should specify the joints the device encompasses and suggest the desired biomechanical alignment and materials of fabrication. When the orthosis is ready, it should be evaluated both off and on the patient to ensure proper fit and function. When these characteristics are achieved, appropriate training of the patient and family on device use should begin. In the majority of patients after TBI, orthotic management, if possible, is best accomplished with use of plastic molded orthoses. They provide more intimate contact, with better distribution of the controlling/corrective forces over a larger area. These devices tend to be more cosmetic and hence better accepted by the patient. The patient is allowed to exchange shoes as long as constant heel height is maintained to avoid altering the dynamic alignment of the device. Plastic materials are lighter and easier to clean than the traditional metal and leather designs. Lack of sensation and fluctuating edema are relative contraindications to the prescription of plastic molded orthoses. If the patient has adequate visual perception, minimal cognitive impairment, and good social support, he or she can compensate for these deficits and receive the added benefits provided by plastic braces. The availability in recent years of a variety of adjustable ankle joints that can be attached to plastic orthoses has eliminated a major disadvantage of these devices.6,13 Adjustments to the biomechanical alignment and fit must be made by an orthotist.
Metal/leather orthoses continue to have a definite place in the treatment of the TBI patient. In most areas, patients are being transferred to rehabilitation programs much earlier than in the past, and the length of stay in the rehabilitation programs has decreased significantly. Predicting the final rehabilitation outcome of these patients early in their rehabilitation program may be difficult. The ability to adjust the biomechanical alignment of the orthosis with simple tools or to convert a controlling force into an assistive one in order to respond to the patient’s needs is an important advantage. Proper biomechanical alignment of any orthosis is critical to the optimization of ambulation.11 Joint malalignment (lack of congruence between orthotic and anatomical joints) creates a discrepancy in motion that ultimately may produce pain, skin breakdown, and other preventable problems, and can and does prevent a borderline walking patient from becoming a functional ambulator. Some patients with better recovery may be able to compensate for inadequate orthotic prescription or alignment, but this ability does not diminish the importance of appropriate orthotic prescription and fit.
Pathological gait in TBI
The mechanism of TBI can produce varied patterns of presentation of residual dysfunction. Head trauma frequently is the result of a high-velocity accident with a coup–countercoup effect of the brain shearing against the rough inner surface of the skull. Multiple injuries are common, and initial diagnosis is difficult. Lifesaving resuscitation efforts often detract from complete examination, resulting in 11% of patients with missed fractures or dislocations and 34% of patients with missed peripheral nerve injuries. The resulting upper motor neuron syndrome and residual dysfunction can affect one side of the body (hemiparesis), two limbs (paraparesis), three limbs, or all four limbs (quadriparesis). According to the presentation, disturbances of the temporal, spatial, kinematic, kinetic, and possibly EMG patterns of gait occur and are well documented.7,14
Although differences occur from patient to patient, some generalities have been demonstrated, including decreased walking velocity with shorter stride length, shorter stance time, and increased swing time for the involved limb.3,14,15 A decrease in weight bearing on the involved limb has been noted as well as a decrease in single support time. The unaffected limb has increased stance time. Stance phase abnormalities include forefoot first or flat foot initial contact rather than heel first. In addition, ankle inversion may occur, causing the lateral border of the foot to contact the ground and producing instability during weight bearing. Incomplete knee extension may be noted, but more commonly hyperextension of the knee is observed, with continued equinovarus deformity of the ankle during midstance. Limited or missing heel contact may be present. During terminal stance, terminal contact can occur early or late, and the pelvis may drop on the contralateral side.1
Inadequate hip and knee flexion during the initial swing phase may result in toe drag. During midswing, insufficient ankle dorsiflexion is a major problem. Inability to perform coordinated hip flexion and knee extension during terminal swing produces a shortened step length, which may be further complicated by an ankle held in plantarflexion.11
From the functional perspective and to facilitate assessment, gait dysfunction can be categorized on the basis of timing with respect to the gait cycle. During stance phase, an abnormal base of support and limb instability may make walking unsafe, energy inefficient, and possibly painful. Inadequate limb clearance and limb advancement during the swing phase interfere with safety and energy efficiency. A comparison of normal gait patterns to patterns exhibited by individuals with hemiplegia demonstrates differences in temporal, kinetic, and kinematic factors and muscle activation patterns across multiple joints. In order to properly identify and evaluate the gait problems of the TBI patient, the clinician must be able to understand what the problem is, when and where it is present, and, if possible, why it occurs. Knowledge of the orthotic devices available to correct the problem, the anatomic limitations of the patient, and a thorough medical, cognitive, and social history are needed to determine the most appropriate orthotic intervention.3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree