37 Key Points 1. Biomaterials are designed in accordance with the type of injury. 2. Biomaterials are either synthesized or derived from natural sources. 3. Biomaterial scaffolds can be designed to promote host tissue regeneration and/or support cell transplantation. 4. Local delivery strategies provide a minimally invasive way to release biomolecules directly to CNS tissue. The complexity of the secondary injury after traumatic spinal cord injury (SCI) has limited the success of clinical treatment regimes with respect to restoring neurobehavioral function. For this reason, much research has been devoted to investigating experimental strategies utilizing a variety of materials to enhance tissue sparing and regeneration following an injury. Tissue engineering involves the improvement, restoration, or replacement of tissue or organs in the human body by manipulating three basic constituents of biological tissues: cells, molecular signals, and extracellular matrices (ECMs). Materials that interface with biological tissues to evaluate, treat, or replace these basic constituents or their function in the body are termed biomaterials.1 Biomaterials comprise a diversity of natural and synthetic materials that are often designed to be biomimetic. The primary requirement for any biomaterial is biocompatibility—that is “the ability of a material to perform with an appropriate response in a specific application.”2 A biomaterial should not cause a significant immune response locally or systemically and must not be appreciably cytotoxic. The safety of a material for in vivo applications is highly dependent on these properties but is also impacted by its size, shape, mechanical properties, and degradation products. Other properties that are important for the choice of a biomaterial are degradation rate, cell adhesion, ease of chemical manipulation for protein or peptide attachment, as well as the ability to process materials into desired shapes with the porosity required for cellular infiltration and macromolecular diffusion. In SCI, the lack of ECM at the lesion site presents a challenge to cell migration and formation of tissue bridges across cavities or gaps that can form in the cord after injury. Biomaterial scaffolds provide a physical substrate on which cells attach and grow, serving as a replacement for the natural ECM during the regenerative process. The type of SCI sustained dictates the type of biomaterial that would be suitable for a given tissue-engineering strategy, and reviews by Nomura et al.,3 Hejcl et al.,4 and Samadikuchaksaraei5 discuss these at length. Full transection and hemisection injury models in which the spinal cord is completely or partially severed are often used in preclinical research because these injuries provide a clear delineation of the injury site, are highly reproducible, and can easily accommodate scaffold implantation. Full transection injuries require a physical support to reconnect the two stumps of the spinal cord; thus biomaterial strategies that have been employed in these models include guidance channels and fiber networks, both of which provide directional cues to enhance tissue regeneration. Hemisection models often have scaffolds implanted6–8 to promote repair, or they combine scaffolds with drug delivery to achieve greater efficacy.9 Compression injuries are the most common type of SCI in humans, and they represent the most clinically relevant model for treatment. However, the injury model does not allow facile implantation of preformed scaffolds because there is not a clear implantation site, thereby necessitating the use of injectable tissue engineering strategies. Most of the research with cavity-forming injuries has been focused on drug delivery and neuroprotective strategies. Polymeric hydrogels are the most commonly used biomaterials for tissue engineering in the central nervous system (CNS) due to their high water content (similar to tissues in vivo) and because the properties of these materials are highly tunable to meet biological requirements. Natural polymers, including polysaccharides and native ECM proteins, are prominent in neural tissue applications. These materials are generally biodegradable and well tolerated in vivo. Because synthetic materials are often easier to manipulate chemically and mechanically, they too have been used extensively for various applications in CNS regeneration. Table 37.1 summarizes commonly used biomaterials for spinal cord repair strategies. Natural biomaterials are derived and purified from biological sources, mammalian or otherwise, and are appealing because they are often found in native mammalian tissue and include collagen, hyaluronan, laminin, and fibronectin. Collagen is a fibrillar protein and the main structural component of most connective tissues in the body. Glycosaminoglycans, such as hyaluronan, chondroitin sulfates, and heparin sulfates, are highly hydrated molecules and ubiquitous in the CNS.10 Other natural biomaterials are derived from plant and nonmammalian sources. Chitosan, a deacetylated form of the polysaccharide chitin (a major component of fungi and arthropod exoskeletons), has been used extensively in the development of guidance channels for spinal cord regeneration.11,12 The degree of deacetylation of chitosan allows degradation rate, cell adhesion, and amount of neurite extension to be tailored.13 Other polysaccharides, including agarose, alginate, methylcellulose, and dextran, have also been investigated as biomaterials in neural tissue engineering (Table 37.1). Synthetic polymeric hydrogels are also of interest because they can be uniformly produced, and their properties easily tuned. Examples of synthetic biomaterials include derivatives of both poly (acrylate) and poly (acrylamide), and notably poly (2-hydroxyethyl methacrylate) (PHEMA) and poly (2-hydroxypropyl methacrylate) (pHPMA), which have been studied as nerve-guidance-channel materials with controlled structure and wall porosity.14 Because surgical removal of nondegradable materials is undesirable, several resorbable synthetic polymers have been investigated for nerve-guidance materials, including polyesters, polycarbonates, and polyurethanes. Most of the synthetic polyesters used in these applications are based on only a few monomer units, used alone or in combination, including poly (glycolide) (PGA), poly (l-lactide) (PLLA), poly (d,l-lactide) (PDLLA), and poly (∊-caprolactone) (PCL). The mechanical and biological properties of these materials can vary widely based on changes in the copolymer composition and fabrication method. For example, scaffolds consisting of PGA or its copolymers with PDLLA or PLLA generally show much shorter degradation times (2 to 12 months) than those composed of PLLA alone or in combination with PCL (up to 2 years). Newer generations of biomaterials incorporate several elements and mechanisms that govern axonal extension and guidance, including physical guidance cues of scaffolds and fiber networks, chemical guidance molecules of cell-signaling molecules, and cell–cell interactions. The following sections describe some of the in vitro and in vivo published literature in these areas and their application to translational neural tissue engineering strategies. Table 37.1 Common Biomaterials Used in SCI Research
Approaches Using Biomaterials for Tissue Engineering
 Biomaterials in Neural Tissue Engineering
Biomaterials in Neural Tissue Engineering
Injury-Specific Biomaterial Design
Biomaterial Sources
Material | Description | Device designs |
Natural materials | ||
Agarose | Thermogelling polysaccharide that is non-cell-adhesive and nondegradable | Hydrogel27 |
Alginate | Polysaccharide that undergoes ionic crosslinking in the presence of calcium | Hydrogel57 |
Collagen | Fibrillar cell-adhesive ECM protein | |
Chitosan | Polysaccharide derived from chitin degradation and cell adhesion properties can be tuned13 | |
Fibrin | Enzymatically polymerized fibrinogen; can potentially be derived from autologous sources; non-cell-adhesive | Hydrogel29 |
Hyaluronic acid | ECM glycosaminoglycan that is injectable and non-cell-adhesive | |
Matrigel | Derived from mouse tumor cells, limited clinical relevance; highly cell adhesive | Hydrogel18 |
Methylcellulose | Reverse thermogelling, degradable polysaccharide that is non-cell-adhesive | |
Synthetic materials (degradable) | ||
PLA/PGA/PLGA | Poly α-hydroxyacids of lactic and glycolic acids; degradation occurs via hydrolysis and can be tuned based on composition | |
PCL | A degradable polyester of ∊-caprolactone; degrades by hydrolysis, but more slowly than PLA | |
PHB | Polyhydroxybutyrate is a biocompatible polyester that degrades by surface erosion but more slowly than PLA | |
Amphiphilic peptides | Can be made to express cell-adhesive peptide sequences, and can self-assemble into nanofibers | |
Synthetic materials (nondegradable) | ||
PHEMA/PHEMA-MMA | Poly (2-hydroxyethyl methacrylate) and copolymers are synthetic hydrogels whose mechanical properties can be matched to the spinal cord | |
PHEMA (NeuroGel, Aqua Gel Technologies) | Porous crosslinked hydrogel used in spinal cord and brain applications | Hydrogel69 |
PAN/PVC | Copolymer of polyacrylonitrile and polyvinylchloride can be used to form stable and nontoxic channels | Channel70 |
Abbreviations: ECM, extracellular matrix; PAN/PVC, poly (acrylonitrile-co-vinyl chloride); PGA, poly (glycolide); PHEMA/PHEMA-MMA, poly (2-hydroxyethyl methacrylate)/poly (2-hydroxyethyl methacrylate-co-methyl methacrylate); PHPMA, poly[N-(2-hydroxypropyl) methacrylamide]; PLA, polylactic acid; PLGA, polylactic-co-glycolic acid.
 Scaffolds for Neural Tissue Engineering
Scaffolds for Neural Tissue Engineering
Physical Guidance Systems for Regeneration
Popularized by their clinical success in treating peripheral nerve injuries,15 guidance channels have been widely investigated in SCI repair. The channel provides a substrate for directed growth while shielding the local area from the surrounding environment, which is inhibitory to regeneration. Guidance channels can be made from synthetic or naturally occurring polymers and can be degradable or nondegradable. Early studies with guidance channels focused on the use of nondegradable materials, such as silicone, poly (acrylonitrile-co-vinyl chloride) PAN/PVC, and poly (2-hydroxyethyl methacrylate-co-methyl methacrylate) (PHEMA-MMA), to ensure stability of the channels; however, biodegradable materials, such as polylactic-co-glycolic acid (PLGA) and chitosan, have become more popular for clinical translation. These materials can be tuned to degrade on the order of months to years, allowing for the relatively long time thought to be required for neural regeneration. Animal studies conducted in rats have used chitosan guidance channels to promote the formation of a tissue bridge between the rostral and caudal stumps as early as 5 weeks after injury,16 whereas animals receiving no treatment fail to form a distinct tissue bridge after 14 weeks.17 Tissue bridges formed inside guidance channels contain numerous axons, indicating that a host neural regenerative response is being stimulated. When combined with cellular therapy, these channels were shown to greatly enhance transplant survival.17
Several groups have incorporated intricate engineering designs to provide greater surface area for tissue ingrowth. This includes matrix-filled channels,18,19 channels within scaffolds,20,21 and aligned fiber networks.22 Moreover, immobilized growth factor patterning23,24 and electrically active materials20 are also being investigated as potential methods for guiding regeneration.
Hydrogel Networks
Hydrogels are crosslinked hydrophilic polymer networks that contain high water content, making them highly attractive for implantation in soft tissue. Hydrogels generally have open porous networks ideal for cell migration and free nutrient exchange between surrounding tissue. However, the most appealing property of hydrogels is that many are injectable or can undergo scaffold formation in situ, facilitating space-filling of irregularly shaped gaps or cavities while minimizing the invasiveness of implantation. A hydrogel strategy for filling a spinal cord cavity is illustrated in Fig. 37.1. The most common biomedical hydrogels include those that are derived from natural polymers, such as chitosan, methylcellulose, collagen, hyaluronan, agarose, alginate, dextran, and fibrin, and those that are synthetic, such as poly[N-(2-hydroxypropyl) methacrylamide] (PHPMA or NeuroGelTM, Aqua Gel Technologies, Inc., Quebec City, Quebec, CA),25 PHEMA, poly (N-isopropyl acrylamide) (pNiPAAm), and polyethylene glycol (PEG).
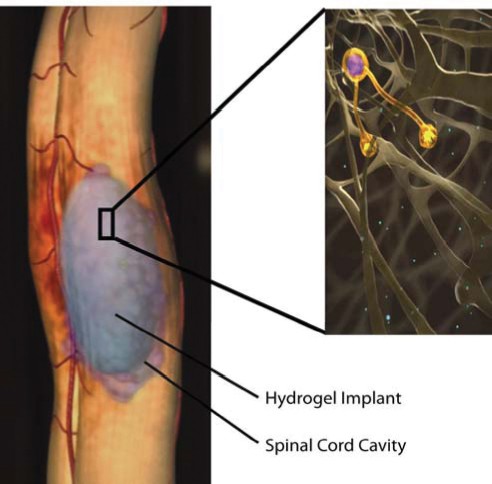
Fig. 37.1 Illustration of a hydrogel implant into a spinal cord cavity showing neurite extension into the scaffold. (Permission courtesy of Hyun-Joo Lee).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


