Fig. 42.1
(Upper) Anteroposterior X-ray of a 75-year-old female patient. Right trochanteric fracture was recognized on radiological findings. (Lower) Surgical treatment with intramedullary nail was undertaken successfully
A 75-year-old female patient came to the hospital by an ambulance with symptoms of the right inguinal and thigh pain after falling down from the standing position. After physical examination and X-ray studies, right trochanteric fracture was recognized (Fig. 42.1). She received surgical treatment with intramedullary nail, the next day after she was admitted to the hospital. T-score of the left femoral neck BMD of the patient was −2.9. At her control visit, after she was discharged from hospital, when she could walk independently, oral tablet of bisphosphonate was prescribed. Under treatment with bisphosphonate, the patient was followed for 2 years.
When the patients have fragility fracture or T-score of BMD below −2.5, medical treatment with antiresorptives or anabolic agents is strongly recommended to prevent the next fragility fractures. In this case, she had low-energy hip fracture from the standing position and T-score was −2.9. The medical treatment for osteoporosis should be started for this case.
Patients with a previous low-energy hip fracture have a relative risk of 9.79 (95 % CI 9.07–10.55) of a subsequent low-energy hip fracture [1]. A relative risk of all low-energy fractures significantly increases for both sexes: 5.55 for men and 2.94 for women.
Bisphosphonates and denosumab have evidence of preventing hip fractures in postmenopausal osteoporotic women on the basis of RCT. RRR of hip fractures is 55 % in alendronate from the results of meta-analysis of six RCTs [2], 40 % in risedronate from one RCT [3], and 40 % in denosumab from one RCT [4]. However, there has been no RCT data showing that SERMs or calcitonin reduce the risk of hip fractures. When the target of treatment is to prevent the subsequent hip fractures, we should choose bisphosphonates or denosumab as the appropriate antiresorptives.
Beneficial effect of risedronate for preventing recurrent hip fracture is reported in the elderly Japanese women [5]. The hazard ratios (95 % CI) obtained by univariate and multivariate analysis were 0.31 (0.12–0.80) and 0.22 (0.07–0.64), respectively, indicating a significantly lower incidence of unaffected side hip fracture in the risedronate group.
The aim of the therapy with antiresorptive medicines (bisphosphonates, SERMs, denosumab, and calcitonin) is to restore BMD by decreasing bone remodeling and to reduce the risk of fractures. After the treatment, we should monitor the effectiveness of the treatment by measuring BTMs and BMD, and by checking that there is no subsequent fragility fractures.
AFFs have been reported in the cases of long-term treatment with bisphosphonates. There are currently no clear conclusions to suggest how long an osteoporosis drug remains safe and effective. Evidence is limited regarding the risk of fractures after discontinuation of long-term treatment with bisphosphonates. The benefits may continue for several years, or the beneficial effect begins to lessen with increased bone remodeling rates. Black et al. [6] describes the one possible recommendation. Patients with low BMD at the femoral neck (T-score below −2.5) after 3–5 years of treatment are at the highest risk for vertebral fractures and therefore appear to benefit most from the continuation of bisphosphonates. Patients with an existing vertebral fracture who have a somewhat higher (though not higher than −2.0) T-score for BMD may also benefit from continued therapy. Patients with a femoral neck T-score above −2.0 have a low risk of vertebral fracture and are unlikely to benefit from continued treatment. After 3–5 years of taking bisphosphonates, individuals should discuss whether they should continue taking the medicine, stop taking the medicine, or consider switching to a different medicine. When a patient has a good response to treatment with bisphosphonates, we can consider a drug holiday.
Introduction
Osteoblasts continuously form new bone while osteoclasts resorb old bone through the remodeling process. A balance of bone formation and bone resorption is usually maintained. An imbalance of bone resorption over bone formation occurs with menopause, aging, and other conditions. An imbalance can result in bone loss that eventually leads to osteoporosis and fragility fractures. Most cases of osteoporosis in postmenopausal women have high turnover of bone metabolism, with enhanced bone resorption over bone formation.
Antiresorptive medicines suppress excessive bone resorption during the remodeling cycle. In general, loss of bone mineral density (BMD) is decelerated, and then BMD is gradually increased at 6 months to 1 year, after the patients with osteoporosis take antiresorptives. The goal of the treatment with antiresorptives is to increase BMD and to prevent the incidence of fragility fractures.
Today, there are three types of osteoporosis medicines. The first is antiresorptive medications, such as bisphosphonates, selective estrogen receptor modulators (SERMs), denosumab [anti-receptor activator nuclear kappa-B ligand (RANKL) antibody], and calcitonin, which suppress bone resorption. The second is anabolic agents that enhance bone formation, such as teriparatide. The third is strontium ranelate, which inhibits bone resorption and enhances bone formation.
Antiresorptive agents for treatment of osteoporosis should be appropriately selected according to the effectiveness and safety, on the basis of meta-analyses, randomized controlled trials (RCTs), authorities’ opinions, and personal experiences.
Antiresorptive Agents
Bisphosphonates
Action
Bisphosphonates are compounds characterized with two C–P binding sites. Bisphosphonates are not dissolved by any enzymes and are difficult to be metabolized. Bisphosphonates at the first generation (e.g. etidronate) do not contain nitrogen at the side chain. Those at the second generation (e.g. alendronate, ibandronate) contain nitrogen at the side chain without cyclic structure. Those at the third generation (e.g. risedronate, minodronate) contain nitrogen at the side chain with cyclic structure.
Bisphosphonates decrease bone-remodeling rate by decreasing osteoclast activity. They diminish ruffled border and induce apoptosis in osteoclasts (Fig. 42.2). This allows more time for secondary mineralization, increases the degree of trabecular mineralization, and mechanically strengthens the bone.
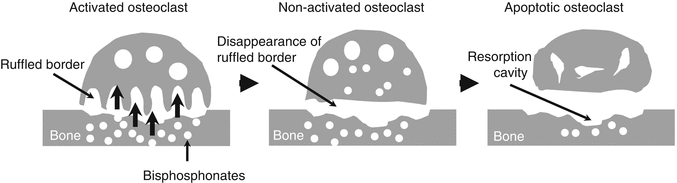

Fig. 42.2
Bisphosphonates have robust affinity to bone matrix and adhere to bone surface. Through the process of bone resorption, bisphosphonates are up-taken into the osteoclasts (left). After ruffled border disappears (middle), apoptosis of osteoclasts is induced (right). In results, bone resorption is suppressed
All kinds of bisphosphonates are approved for the treatment of osteoporosis as well as for the treatment of glucocorticoid-induced osteoporosis. Bisphosphonates can be dosed orally––daily, weekly, or monthly, or intravenously––every month, every 3 months, or annually. Because of the difficulty of gastrointestinal absorption, the oral tablets of bisphosphonates need to be taken early in the morning and on an empty stomach, at least 30 min before having anything to eat or drink except water. The tablet is swallowed with at least one cup of water. Patients must not be lying down during the 30 min, after taking the medicine.
Alendronate
Alendronate increases BMD by 13.7 % at the lumbar spine and by 5.4 % at the femoral neck in 10 years [7]. Alendronate reduces the fracture risks of the vertebral, hip and other bones by about 50 % over 2–4 years. The drug is well-tolerated over a 10-year period. Relative risk reduction (RRR) for a 10-mg dose of alendronate in meta-analysis with 11 trials representing 12,068 women is as follows [8]. For vertebral fractures, significant 45 % RRR is found [95 % confidence intervals (CI) 0.45–0.67]. This is significant for both primary prevention with 45 % RRR (95 % CI 0.38–0.80) and secondary prevention with 45 % RRR (95 % CI 0.43–0.69). For nonvertebral fractures, significant 16 % RRR is found (95 % CI 0.74–0.94). This is significant for secondary prevention with 23 % RRR (95 % CI 0.64–0.92), but not for primary prevention (RR 0.89, 95 % CI 0.76–1.04). There is significant 40 % RRR in hip fractures (95 % CI 0.40–0.92), but only secondary prevention is significant with 53 % RRR (95 % CI 0.26–0.85). The only significance found for wrist is in secondary prevention with 50 % RRR (95 % CI 0.34–0.73). According to the results of Fracture Intervention Trial (FIT), the number needed to treat (NNT) of alendronate against vertebral fractures in postmenopausal osteoporotic women is 14.3 [9].
Risedronate
Meta-analysis reveals that risedronate increases BMD by 4.54 % at the lumbar spine and by 2.75 % at the femoral neck in 1.5–3 years [10, 11]. RRR in meta-analysis with seven trials representing 14,049 women for risedronate 5 mg dose is as follows [12]. Risk estimates for primary prevention are available only for vertebral and nonvertebral fractures and show no statistically significant effect of risedronate on fractures. For secondary prevention, significant 39 % RRR in vertebral fractures (95 % CI 0.50–0.76) is found. For non-vertebral fractures, significant 20 % RRR (95 % CI 0.72–0.90), and for hip fractures, there is significant 26 % RRR (95 % CI 0.59–0.94). When primary and secondary prevention studies are combined, the reduction in fractures remains statistically significant for both vertebral (RR 0.63, 0.51–0.77) and nonvertebral fractures (RR 0.80, 0.72–0.90). According to the results of Vertebral Efficacy with Risedronate Therapy––North America (VERT-NA), NNT of risedronate against vertebral fractures in postmenopausal osteoporotic women is 20.0 [9].
Other Bisphosphonates
Ibandronate, minodronate, and zoledronate are promising osteoporosis treatment modalities. Their molecular and cellular action mechanisms are substantially the same as those of alendronate and risedronate, which are described in section “Action”. The bone mineral affinity is different among bisphosphonates. The percentages of the fraction bound to bone in vivo after dosing are 40–50 % in ibandronate, 50–62 % in zoledronate, 60 % in risedronate, and 64 % in alendronate [13].
Ibandronate reduces the incidence of vertebral fractures by about 50 % over 3 years. In the USA, ibandronate is taken once monthly as a 150 mg tablet, and it is also available as an intravenous (IV) injection of 3 mg given every 3 months. In Japan, it is available as an IV injection of 1 mg given every month. Medical doctors or nurses have to administer the IV dose in a clinic or a hospital. It takes less than a minute to inject.
Minodronate is developed in Japan. It is the most robust inhibitor of farnesyl pyrophosphate synthase among the bisphosphonates in vitro [14]. Minodronate increases BMD and shows 59 % RRR at vertebral fractures in 2 years [15]. According to the results of the phase III study in Japan, NNT of minodronate against vertebral fractures in postmenopausal osteoporotic women is 7.4 [9]. Minodronate is indicating good efficiency of treatment.
Zoledronate is available in the USA. It is given once a year as an IV infusion to treat osteoporosis. Before approval for use in osteoporosis, zoledronate was already available for use in cancer patients with certain bone conditions. It increases BMD by 6.71 % at the lumbar spine, 6.02 % at the total hip, and 5.06 % at the femoral neck over the respective values of the placebo group [16]. It also shows 70 % RRR at vertebral fractures and 41 % at hip fractures in 3 years. Zoledronate shows significant 35 % RRR for any new clinical fractures and significant 28 % RRR for death from any causes [17]. Less frequent administration may lead to better adherence and efficacy.
Etidronate is the first generation bisphosphonate and does not contain nitrogen at the side chain. Recently, etidronate is not very often used clinically. RRR in meta-analysis with 11 studies representing a total of 1248 patients is as follows [18]. Significant 41 % RRR in vertebral fractures across eight studies (95 % CI 0.36–0.96) is found. For vertebral fractures, the six secondary prevention trials demonstrate significant RRR of 47 % (95 % CI 0.32–0.87), but the pooled result for the two primary prevention trials (RR 3.03, 95 % CI 0.32–28.44) is not significant. There are no statistically significant risk reductions for nonvertebral (RR 0.98, 95 % CI 0.68–1.42), hip (RR 1.20, 95 % CI 0.37–3.88), or wrist fractures (RR 0.87, 95 % CI 0.32–2.36).
Side Effects
Side effects of the oral tablets for all bisphosphonates may include gastrointestinal disorders such as irritation of the esophagus and gastric ulcer. When the patients receive monthly oral tablets or IV bisphosphonates, flu-like symptoms such as fever, headache, and pain in muscles or joints may occur. These generally disappear within a few days without any treatments and usually do not appear on further administrations.
There have been rare reports of osteonecrosis of the jaw (ONJ) after invasive oral treatment in the patients treated with bisphosphonates. The prevalence of ONJ in osteoporosis patients is 0.028–4.3 % (about 1/100,000 person-year) [19], though cancer patients receiving intravenous bisphosphonates at much larger doses than given for osteoporosis have higher risk at odds ratio of 4.27 (95 % CI 3.38–5.40) than oral bisphosphonates [20].
Atypical femoral fractures (AFFs) in the patients taking bisphosphonates for a long term have been reported. Most patients have thigh or inguinal pain for several weeks before fractures. The findings of fractures on radiographs are characteristic. They are described in detail by a task force of the American Society for Bone and Mineral Research [21]. The prevalence of AFFs in osteoporosis patients is 32–59/1,000,000 person-year.
SERMs
Action
SERMs are neither estrogens nor hormonal agents. SERMs suppress bone remodeling into the premenopausal range, while keeping the function of osteoblasts and osteocytes. SERMs suppress bone resorption within the physiological levels by enhancing the production of osteoprotegerin, decoy receptor of RANKL [22].
SERMs exhibit tissue-specific activity. They are estrogen agonists/antagonists that provide the beneficial effects of estrogens without their potential disadvantages. The selective actions of SERMs in different tissues result from a combinatorial collaboration among several factors [23]. Estrogen and SERMs, all bind to estrogen receptors, inducing distinctly different receptor conformations. These different receptor conformations then interact with the regulatory sequences of target genes in different ways. The type of interaction and the cellular levels of co-regulator proteins (co-activators or co-repressors) determine the distinct patterns of co-regulator recruitment to the ligand–receptor–gene assembly. In this way, either stimulation or inhibition of specific biological effects is elicited.
SERMs act as estrogen agonist in bone and lipid metabolism, while they act as antagonist in female sexual organs such as breast, uterus, and ovary. Substantially, raloxifene therapy shows a favorable or neutral effect in the cardiovascular system and reduces the incidence of estrogen receptor-positive breast cancer [24]. Raloxifene and bazedoxifene are available in Japan. They are approved for the treatment of postmenopausal osteoporosis.
Raloxifene and Bazedoxifene
The evidence supports the efficacy of raloxifene or bazedoxifene for the prevention of osteoporotic fractures. The published systematic reviews did not include other SERMs such as lasofoxifene, ormeloxifene, and femarelle.
Raloxifene and bazedoxifene increase BMD and reduce the risk of vertebral fractures. There are no data showing that raloxifene and bazedoxifene reduce the risk of hip and the other nonvertebral fractures, excluding the post hoc analyses. Raloxifene is taken daily as a 60 mg tablet and bazedoxifene is taken daily as a 20 mg tablet with or without meals. Side effects include hot flashes, leg cramps, and deep vein thrombosis.
Denosumab
Action
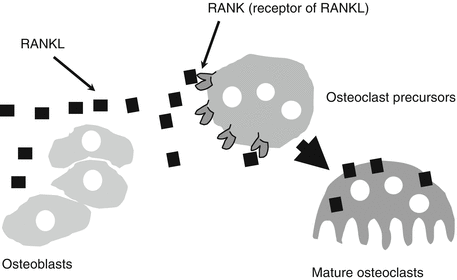
Denosumab is a RANKL inhibitor and human monoclonal antibody. RANKL, a key molecule of osteoclast differentiation, is produced by osteoblasts and osteocytes (Fig. 42.3). RANKL production is increased in postmenopausal osteoporotic women and stimulates osteoclast differentiation to destroy bone.
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








