Chapter 22 An International Perspective on the Foot and Ankle in Sports
A. Personal Perspective on Foot and Ankle Sports Conditions
Ankle Instability
The lateral ligamentous complex of the ankle may be the most commonly damaged structure in sport injuries.1,2 Garrik3 reported a frequency of 45% in basketball practice, 31% in soccer, and 25% in volleyball.
Primary repair of the ligaments was previously recommended; nonoperative treatment also has been recently recommended.2,4 However, despite adequate primary functional treatment, some patients develop chronic instability. In 20% of the cases, ligamentous reconstruction is required.5 Indications for ligament reconstruction are mechanical and functional instability and failure of rehabilitative treatment. The goal of surgical treatment is to improve stability and proprioceptive sensation maintaining complete range of motion (ROM).
Ankle instability surgery has been divided into an anatomic repair consisting of imbrications of the local tissue of the lateral ligamentous complex and an ankle-ligament reconstruction involving tendon grafts. A nonanatomic tenodesis results in stiffness of the operated ankle, prolonging recovery and decreasing sport6 because of the incorrect orientation of the reconstructed ligaments.
Surgical technique 1
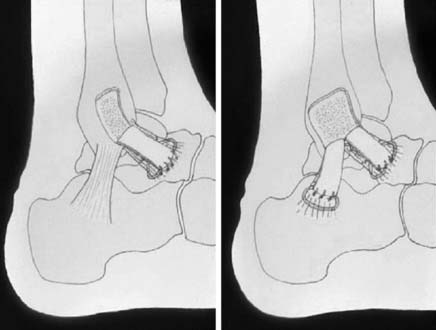
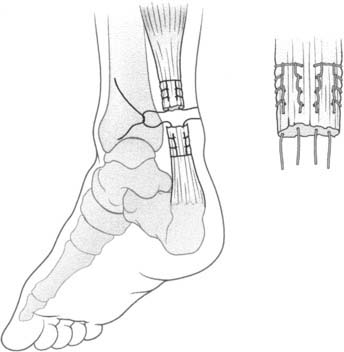
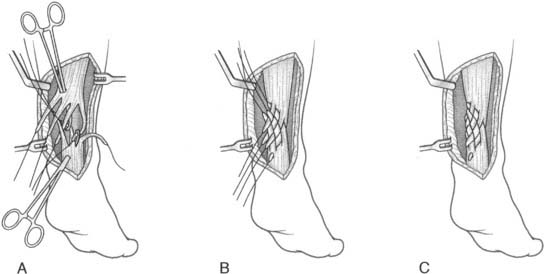
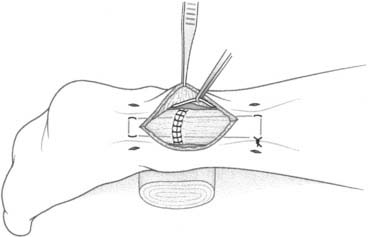
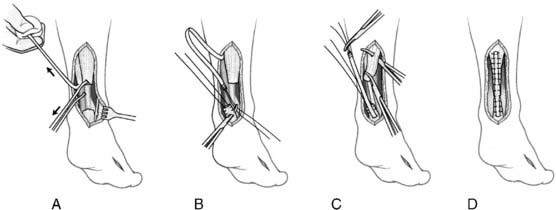
The anatomic reconstruction has been described previously by Brostrom4 and consists of the direct suture of the stumps of residual tissue. In our experience, to reinforce the reconstructed ligaments a periosteal flap should be harvested from the anterolateral aspect of the fibula and turned down and sutured as talofibular ligament or split in two and used also to reinforce the calcaneofibular ligament (Fig. 22A-1).
Surgical technique 2
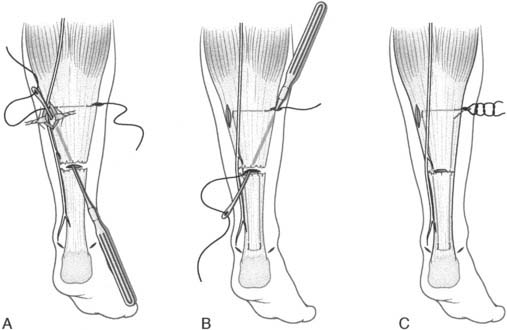
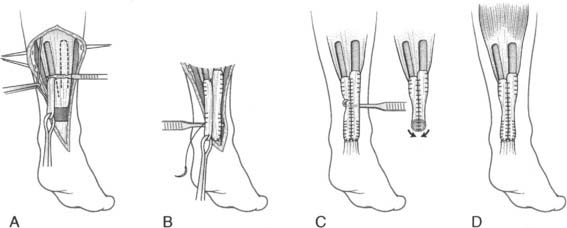
When the residual tissues are not strong enough to permit direct suture or after failure, we perform a reconstruction of the talofibular and calcaneofibular ligaments using the plantaris, if present, or a cadaveric tibialis posterior graft. The tendon is fixed through a transosseous tunnel or with an anchor on the neck of the talus. A tunnel is created through the anterior aspect of the lateral malleolus where the talofibular is inserted and the tendon is passed in and sutured to the periosteum. In case of an associated calcaneofibular lesion, the tendon will be passed through the apex of the lateral malleolus and be sutured on the lateral wall of the calcaneus (Fig. 22A-2). We pay particular attention to reconstruct the ligament with a proper length, direction, and tightness similar to those of the healthy anatomic complex to obtain an isometry of the new ligaments permitting a physiologic ROM and avoiding stiffness.7
In a 1983 study, Giannini et al.8 concluded that 67% of ankle sprains in sports activity were in athletes with cavus foot. Because of this observation, in cases with a cavus foot associated with a varus of the calcaneus, evaluated as reducible according to the Coleman test,9 a mini-invasive dorsiflexion metatarsal osteotomy (see Fig. 22A-2) associated with the ligamentous reconstruction is indicated. This procedure will rebalance the foot, helping to prevent further sprains and improving function.
Osteochondral Lesions of the Talar Dome
Osteochondral lesions of the talar dome are very common in sports activity as a consequence of ankle sprains.10,11 Procedures for the treatment of osteochondral lesions of the talus including debridement of the joint, shaving of fibrillated cartilage, and resection or perforation of subchondral bone in the last decade have been performed arthroscopically with low morbidity. These surgeries are not effective in lesions larger than 1.5 cm2 and have not been histologically effective in restoring the hyaline cartilage.12–20
Autologous chondrocyte transplantation (ACT) has proved to be capable of restoring the articular hyaline cartilage surface, including defects larger than 2 cm2 (Figs. 22A-3, 22A-4, and 22A-5).17 In the past, this practice required a medial or lateral malleolar osteotomy, and, although there were good clinical and histologic results, the technique was quite invasive and technically demanding.17 Recently, advancement in tissue engineering permitted the development of absorbable synthetic scaffolds, permitting a completely arthroscopic technique through the traditional anteromedial and anterolateral approaches. Because of this improvement, it appears to be reasonable, mostly in the young athletes, to extend the indications of ACT even in smaller lesions traditionally treated with microfractures.
Achilles Tendon Lesions
Achilles tendon lesions in soccer are 31% to 34% of all traumas, according to Lanzetta et al.21 The Achilles tendon rupture usually is caused in soccer by direct or indirect trauma during jumping, cutting, or turning.22 Predisposing factors in the soccer player are due to an overuse of the calcaneal-Achilles-plantar system, possibility of preexisting tendinopathy, or corticosteroid injections.
The clinical presentation is variable. Pain may be mild for the preexisting degeneration of the tendon because functionality may be performed by the retromalleolar pronator and supinator muscles with different percentages, making the clinical evidence less clear. Rerupture occurs in 10% to 30% of high-performance active patients with nonoperative treatment;23–25 therefore surgery generally is recommended. Because formal open procedures have been associated with a high rate of complications related to poor wound healing, deep infection, adhesion of scar tissue, and disturbance of sensation,23,24 our choice is surgical repair with a mini-invasive technique. The advantages of mini-invasive surgery are less surgical trauma, better quality of reparative scar tissue, avoidance of damage to the local vascularity, faster recovery, and return to sport activity. Indications for the mini-invasive treatment are lesions from 6 to 8 cm from the calcaneal insertion and no more than 6 days after the rupture.
Surgical technique
The Achilles tendon repair system (Fig. 22A-8) permits a suture of the tendon through a 1.5-cm incision. Both stumps of the ruptured tendon are identified. The instrument is introduced in the closed position, under the paratenon, in a proximal direction. When the tendon lies between the two branches of the instrument, the sutures are passed, and the end of each is held with a small clamp to keep the sutures separate from each other. When the instrument is withdrawn, the sutures slide to a peritendinous position. Afterward, the same sequence is performed on the distal stump, and the tendon reduction is performed under visual control.
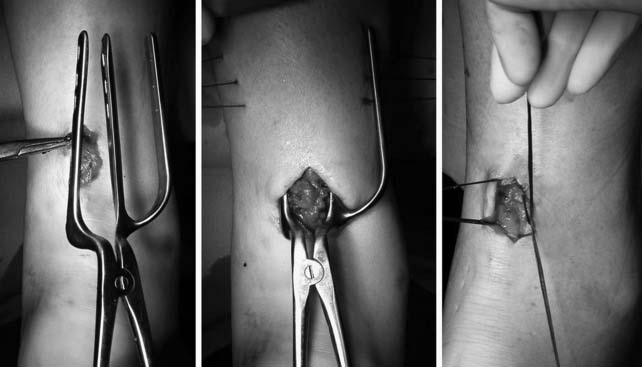
Figure 22A-8 The Achilles tendon repair system permitting a suture of the tendon through a 1.5-cm incision.
Plantar Fasciitis
Plantar fasciitis is common in high-performance athletes, mostly runners and basketball and volleyball players because of the high stress concentrated at the fascia insertion in running and jumping.26
Commonly cited risk factors for plantar fasciitis are the flat or cavus foot, a tight Achilles tendon, the type of training shoes worn, and errors in the training routine.27
A safe and effective nonoperative treatment that we feel should be considered before surgery is the application of low-energy shock waves at the fascia insertion (three applications of 2100 impulses of low-energy shock waves), usually providing good results.28 If the fasciitis does not respond to the nonoperative treatment, in a minimum of 4 months for a professional athlete, surgical treatment should be attempted.
Because the open technique has a high failure rate, with 15.5% of the patients reporting dissatisfaction,29 we prefer the use of a percutaneous fasciotomy. It is important to note that this technique does require surgical experience and may be associated with complications.
Surgical technique
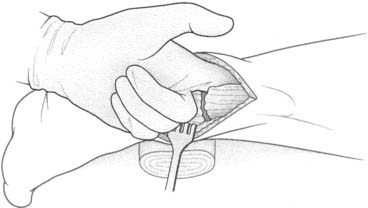
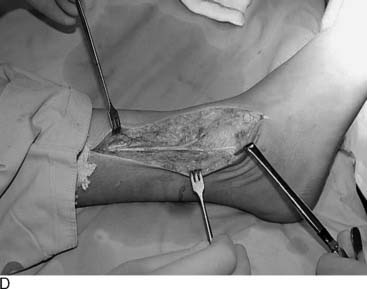
A 14-mm K-wire is inserted manually in the medial aspect of the foot to identify the level of the insertion of the fascia. The fasciotomy is performed with a tenotomy blade while the foot is maintained in dorsiflexion and the fascia is probed externally with a finger (Fig. 22A-9).
Lisfranc Sprains
Injuries to the Lisfranc ligament complex in the general population are uncommon and typically occur as a result of high-velocity and indirect trauma that causes an obvious displacement and disruption of the tarsometatarsal anatomy.30 Low-velocity Lisfranc sprains also can occur after an indirect trauma when the foot is plantarflexed and slightly rotated. This is a frequent condition in soccer players.
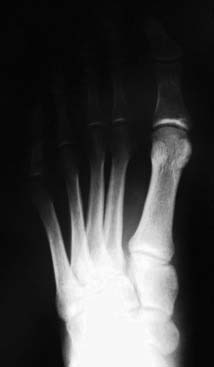
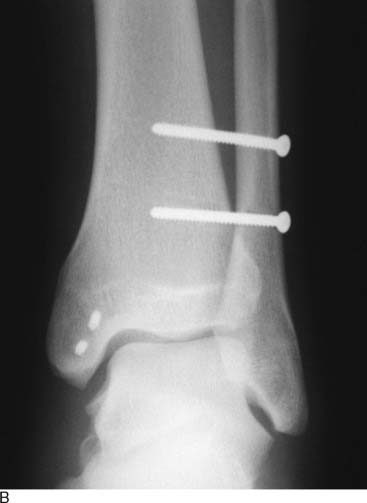
Lisfranc sprains represent a spectrum of injuries to the Lisfranc ligament complex, from partial sprains with no displacement to complete tears with frank diastasis31 (Fig. 22A-10). Although the nondisplaced injuries often heal uneventfully, patients with displacement should undergo a closed reduction and internal fixation with cannulated screws.
Surgical technique
A percutaneously placed large bone clamp is used to assist the reduction. Under C-arm control, percutaneous guidewires are inserted, followed by placement of cannulated screws (Fig. 22A-11).
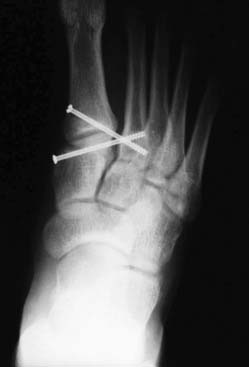
Figure 22A-11 Under C-arm control, percutaneous guidewires and cannulated screw are used to maintain the reduction.
1 Burks RT, Morgan J. Anatomy of the lateral ankle ligaments. Am J Sports Med. 1994;22:72.
5 Renstrom PA. Persistently painful sprained ankle. J Am Acad Orthop Surg. 1994;2:270.
7 Leardini A, et al. A geometric model of the human ankle joint. J Biomech. 1999;32:585.
22 Hattrup SJ, Johnson KA. A review of ruptures of the Achilles tendon. Foot Ankle. 1985;6:34.
25 Roberts C, et al. Dynamised cast management of Achilles tendon ruptures. Injury. 2001;32:423.
27 Warren BL. Plantar fasciitis in runners. Treatment and prevention. Sports Med. 1990;10:338.
30 Mantas JP, Burks RT. Lisfranc injuries in the athlete. Clin Sports Med. 1994;13:719.
B. Treatment of Achilles Tendon Ruptures
Introduction
The rapidly growing trend for participation in recreational and competitive sport is accompanied by an increase of overuse syndromes. In the foot and ankle, the incidence of Achilles tendon rupture and subsequent problems has increased significantly in recent decades.1–3 In Germany the incidence of acute Achilles tendon rupture is estimated to be 15,000 cases/year.3 The rupture usually does not occur at the time of top-level sporting activities. Most studies show a peak between the ages of 30 and 45 years.4–9 The patient collective has a remarkably large portion of leisure-time athletes and patients with sedentary occupations.10 The portion of injuries in track-and-fields athletics is cited as only 10%. These are mostly young patients who sustained a tendon rupture as a result of an incompletely treated achillodynia or an enormous training workload.9 In the future, an increasing number of older patients (older than 50 years) will sustain Achilles tendon rupture as strenuous sports activities become more and more common in this age group. Of all the tendons of the human body, the Achilles tendon seems to be the most susceptible to degenerative changes. The male-to-female ratio of persons with Achilles tendon rupture ranges between 5:1 and 10:1 in most studies, and on average the men are older.9,11 According to the literature and our experience, Achilles tendon ruptures occur most often (in 80% to 90% of cases) 2 to 6 cm proximal to the calcaneal insertion.9 The incidence of proximal ruptures distal to the musculotendineal transition is 10% to 15% and is caused by degenerative changes. Ruptures near the calcaneal insertion are rare and mostly are found in hyperpronators with a heel spur (Haglund’s heel). In contrast to impulsive injury mechanism in tendinous ruptures, bony avulsions usually are caused by continuously increasing tension and strength or direct impact.9 The rupture mechanism usually is a consequence of an indirect loading and traction mechanism, such as a push-off with the foot in plantarflexion and simultaneous knee extension or a sudden, unexpected dorsiflexion of the ankle with powerful contraction of the calf muscles.9 Direct impact, such as a kick or hit on the tensed tendon, accounts for only 1% to 10% of ruptures.11,12 The degenerative and the mechanical theory of etiopathogenesis of Achilles tendon rupture face each other. Aseptic inflammations (tendinitis, paratendinosis) and reduced vascular supply lead to degenerative changes with cell loss and disorders of mucopolysaccharide content, even to fatty, mucoid, or calcifying degeneration.13 Repetitive or single stresses result in minor microtrauma. Low temperature and fatigue of athletes (lactic acid) lead to decreased maximal load resistance.9 If regenerative healing processes cannot keep pace, the sum of microtrauma leads to rupture.
Diagnostics
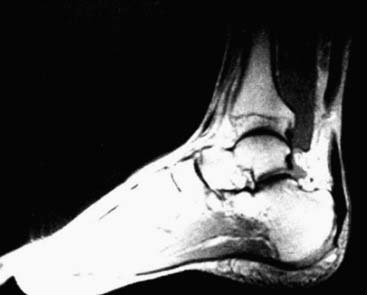
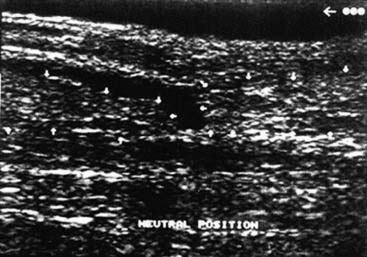
Although most Achilles tendon ruptures can be diagnosed clinically, evaluation by ultrasonography and magnetic resonance imaging (MRI) enables a definitive diagnosis and is decisive for the choice of treatment (Figs. 22B-1 and 22B-2). Ultrasonographic appearance of acute Achilles tendon rupture shows broad variations. The most common signs are interruption of continuity and demarked tendon stumps. Hypoechogenic accumulations of liquid at the rupture site and loss of the typical parallel hyperechogenic reflex patterns are depicted regularly by experienced examiners. Because some ruptures do not show a visible diastase of the stumps from the hematoma, dynamic examination in dorsiflexion and plantarflexion is essential. Even if there is no visible gap, a spreading of fine parallel echoes, corresponding to a loss of cross-wise network of elastic fibers, reveals a rupture. Inflammatory tendinosis with edematous dissolution of the structures must be differentiated. Disrupted or retracted soleus fibers, which are detected mostly in top-level athletes, are significant for the choice of treatment and especially for the surgical technique. Although this can be detected by ultrasonography, MRI shows a better validity.9 The soleus muscle must be examined with sagittal and axial scans. Furthermore the differentiation of rupture area and tendon ends enables an exact determination of the diastase and distance to the calcaneal insertion.
Treatment
Conservative treatment
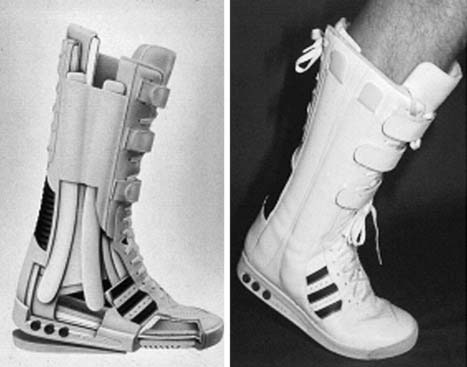
Primary conservative immobilizing treatment and postoperative aftercare in a cast are not justified concerning the disadvantages of muscle atrophy and loss of coordination and proprioception. The concept of primary functional treatment considers the ultrasound or MRI morphology as a basis for treatment strategy. The ultrasonographic or MRI depiction of complete adaptation of tendon ends in 20-degrees plantarflexion is required. The validity of this method, compared with operative treatment, could be proven in a series of more than 550 patients using a high-shaft shoe, comparable to a modified boxer boot (Variostabil, Orthotech, Germany)*14 (Fig. 22B-3). Indication for primary functional treatment independent of the ultrasonographic or MRI findings should be preferred in the elder nonactive patient or in patients with altered operative risk or reduced capacity for tissue regeneration (e.g., after organ transplantation surgery, systemic corticosteroid treatment, diabetes).15
Operative treatment
Issues that comprise the decision for operative treatment include the following:
Techniques
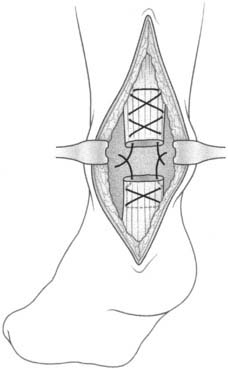
In acute Achilles tendon ruptures, simple end-to-end or three-bundle sutures have been the methods of choice to date (Fig. 22B-4). In recent years in the United States the suture technique by Krackow (Fig. 22B-5) has become popular because it provides strong mechanical stability that allows early functional rehabilitation.16,17 A biomechanical study by Watson et al.,17 however, proved the weak stability of the suture realized by the different open techniques and questioned the advantages of the open surgical treatment.
The combination of the advantages of the biology of tendon healing from the primary functional treatment along with minimally invasive surgery to stabilize the tendon stumps adaptation for the first healing period is addressed by the percutaneous technique described by Buchgraber and Pässler18 (Fig. 22B-6). Using only five small incisions, a 1.3-mm polydioxanone suture (PDS) is guided percutaneously by means of an awl. It connects the proximal tendon with the calcaneal insertion and crosses the rupture site, thereby acting as an internal fixator. To tighten the cord into the tendon, multiple dorsiflexions of the foot are performed. Another advantage of this technique is the remaining integrity of the paratendon, which is important for the healing process. To prevent the potential risk of injuring the sural nerve, an endoscopically assisted percutaneous technique with a 2.8-mm arthroscope can be used.9
The lace technique by Segesser pays special attention to the rotation of radiating tendon bundles, as described by Cummins. With his technique he provides an adequate reinsertion of the medial gastrocnemius and soleus fibers, which often are disrupted or retracted in Achilles tendon ruptures in top-level athletes (Fig. 22B-7).
Treatment of reruptures
In cases with a tendon gapping, a shortening of the gastroc-soleus-Achilles complex with adhesions is probable. This happens in the majority of delayed cases. In these circumstances, a small medial incision (4–5 cm) at the former incision is made. Then the gastro-soleus complex is released distally (Fig. 22B-8). A normal subcutaneous suture is performed and serves as an “internal fixator” of the ruptures tendon. In addition, classic Krakow sutures are applied for the tendon stumps (Fig. 22B-9).
The aftertreatment has the same protocol with the Variostabil boot.
Treatment of chronic ruptures
The problem of chronic rupture is retraction of the tendon stumps with the lack of an efficient regenerate. Sometimes a primary reconstruction is possible, but in most cases reconstructive techniques are indispensable. The decision for the correct reconstructive technique depends on the amount of insufficient tissue. Therefore evaluation by MRI is mandatory. Defects of 2 to 5 cm are the indication for reconstruction with a modified “two flaps technique,” first described by Thermann in the year 2000. For reconstruction, two flaps of the aponeurosis of the triceps surae muscle are used. In the first step, the muscle is released proximal by a medial incision, followed by the preparation of the two flaps from the medial and lateral part of the aponeurosis. Essential for the modification is the turning down and 180-degree rotation of the medial flap approximately 1.5 cm proximal to the corresponding lateral part. This offset considerably facilitates the skin closure later. After fixing the flaps medially and laterally at the distal stump, suturing is performed continuously with a 3.0-mm PDS cord in a “tubulation technique,” thus creating a “neotendon” as a consequence (Fig. 22B-10). The neotendon should be stretched in a manner that forces a slight plantarflexion. For wound healing, a cleaved cast is applied, followed by rehabilitation in the Variostabil boot for 8 weeks according to primary functional treatment.9,19
Reconstruction of larger defects requires a transfer of the flexor hallucis longus tendon20 or the peroneus brevis tendon.21 In both techniques the distal and proximal stump are sewn together with the transferred tendon. Also, the neotendon should be adequately stretched to put the foot in an equine position (Fig. 22B-11). Because the peroneal tendon is not “in phase,” there are only very limited indications for this procedure. Rehabilitation protocol corresponds to the “two flaps technique.”
Rehabilitation
This boot has a plastic tongue to prevent dorsiflexion; the lateral shaft-stabilization reduces torsion, and the reducible heel pad allows a gradual adjustment of 20 degrees from plantarflexion to neutral position. Its functional potential regarding gastrocnemius activities was proved by electromyography, which showed comparable amplitudes to the uninjured side after 3 months.15 With the fitted boot the patient is allowed to perform full weight bearing and to continue the previously begun isometric exercises. The patient wears the boot for 6 weeks, day and night (or alternatively uses a night splint to protect the tendon) and for the following 2 weeks only during the daytime. After 3 weeks the patient is allowed to exercise on a stationary bike, but only with little application of power. In ambitious patients, a physiotherapeutic treatment with well-dosed strengthening exercises (isometric exercises, isokinetic bicycle), proprioceptive neuromuscular facilitation (PNF), and coordination exercises in the boot is allowed after 4 weeks. In addition, ultrasound application (1 Hz) and cryotherapy are performed to enhance tendon regeneration. From the sixth week on, leg-press training is begun in the boot. After 8 weeks, an ultrasonographic control evaluates the restoration of continuity and tendon regeneration. After an appropriate tendon regeneration has been achieved (8 to 12 weeks MRI or sonography control), the treatment in the boot is discontinued. A small heel lift in the normal shoe is recommended for a further 6 to 8 weeks. Jogging is allowed after 3 months if coordination and muscle power are appropriate.
1 Christensen J. Rupture of Achilles’ tendon. Acta Chir Scand. 1953;106:50.
2 Schönbauer HR. Diseases of the Achilles’ tendon. Wien Klin Wochenschr. 1986;14(suppl 1):23.
3 Thermann H. Treatment of Achilles’ tendon ruptures. Foot Ankle Clin. 1999;4:773.
9 Thermann H. [Treatment of Achilles tendon rupture]. Unfallchirurg. 1998;101:299.
C C. Foot and ankle injuries in United Arab Emirates sports
M. Kazim
Certain niche sports are found in the UAE. For example, falcons are trained to hunt prey. This involves long periods of bonding and progressive conditioning of the predator bird. To accomplish this, the owner often has to run rapidly to tend to his falcon, and in the soft sand this is better accomplished barefoot. Sandals typically are worn (Fig. 22C-1) to keep the foot high off the ground to prevent entry of pebbles but are discarded when running in soft sand. Still, minor stub and barb injuries are common. Because the terrain underfoot is soft, the barbs or other objects have little force for any penetration. When training the falcons on the harder desert plain, one wears enclosed shoes (Figs. 22C-2 and 22C-3) to prevent the entry of foreign objects. Also, ankle sprains (mostly lateral) tend to be relatively minor on the sand because the surface is not firm and thus is very forgiving during inversion.


Figure 22C-2 On the harder desert plain, the hunter seen here with the falcon (A) uses enclosed shoes (B).



Figure 22C-3 Another example of the falcon trainer (A) with typical shoewear (B) used for hard desert terrain (C).
Motocross has its share of injuries because dirt bikes are ridden in the sand at high speeds. Riders are required to wear body armor and protective footwear (Fig. 22C-4). However, unlike hard dirt terrain that causes a violent plantarflexion force of short duration, sand produces a more moderate force of longer duration. This leads to sprains of the anterior structures, with relatively frequent anterior capsular tears. Conservative care with walker-type removable braces allows rapid return to riding. Occasionally, more severe problems such as Lisfranc injuries and subluxations or dislocations of the talocrural joint occur. When surgical reconstruction is warranted, rigid internal fixation is used, possibly including the repair of a deltoid ligament avulsion and concomitant syndesmosis stabilization (Fig. 22C-5).
D. Nerve injuries Complicating Inversion Ankle Sprains
Inversion injury imposes stretch and stress also on the more superficial structures, the nerves and integument. Skin swelling and hematoma formation are caused by injury to the skin and its lymphatics, small venules, and capillaries (in addition to bleeding from the torn ligaments) and is a sin qua non of ankle sprain. It usually resolves with time and is not a reason for concern to the treating physician or coach. However, there often is less awareness of the existence and importance of stretch to the nerves.1
Anatomy
The peroneal branch of the sciatic nerve separates from the tibial one at the popliteal fossa, where it takes a more lateral course. It emerges from underneath the biceps tendon near its insertion to fibula head and courses around the neck of the fibula, where it divides to the SPN and deep peroneal nerves (DPN). The SPN travels in the lateral compartment underneath the peroneus longus and exits the crural fascia to become subcutaneous about 10 to 15 cm proximal to the tip of the lateral malleolus in most cases.2,3 There are variations, however, in the exit mode, some of which carry special clinical relevance. The nerve can have a low exit point (5 cm from the tip in 2% and 7.5 cm in 5%).4 It also may penetrate into the anterior compartment first and then through the crural fascia. The SPN bifurcates to main two branches, the intermediate dorsal cutaneous nerve, supplying the dorsolateral aspect of the foot, and the dorsomedial cutaneous branch, which innervates the skin on the medial aspect of the dorsal forefoot and the hallux. Occasionally it also supplies the second toe and some cross innervation with the DPN in the first webspace.3
Pathoanatomy of nerve injury with ankle sprain
Normal excursion of the peroneal nerve during ankle inversion is about 4 cm.5 This excursion is transferred and shared by the whole nerve up to the level of the common peroneal nerve through several gliding mechanisms. Severe ankle inversion may stretch the nerve beyond its physiologic capability to withstand stretch and gliding.6 Anatomic variations and preexisting conditions may hamper the gliding mechanisms and predispose to more severe injury.7 The exit level is important because if there is impedance to nerve gliding through the fascial hiatus and the exit is low, the same stretch is imposed on a shorter nerve segment. Even with a normal exit, overstretching because of severe inversion-plantarflexion may result in nerve damage. Typically a combination of the two will result in a more severe injury. Nerves that penetrate to the anterior compartment before emerging through the fascia cruris also are prone to stretch injury.8
We assume that muscle swelling and increased intracompartmental pressure created during rigorous athletic activity9 presses the nerve against the fascia cruris and impedes nerve gliding through the hiatus. Another hypothesis is the “intraperoneal entrapment.” During acute inversion the nerve glides distally. At the same time, rigorous contraction of the peroneal muscles may compress and entrap the SPN, which courses between them. The contracting muscles pull the nerve proximally, in the opposite direction, thus increasing the stretch on the nerve.
Histologically, in the severe cases stretching injury to nerve will result in perineural tears, which may lead to intraneural and perineural fibrosis.10,11 In cases in which we had to resect the nerve following inversion ankle injury, we saw on histology laceration and discontinuation of nerve fibers. In one extreme case we observed fatty degeneration with marked thickening of the nerve. Macroscopically the picture varies from a nerve that appears normal to a thickened one. The fascial exit site may show frank cicatrization. Extensive scarring may be seen at the nerve bed at the dorsum of the foot, which interferes with nerve gliding (Fig. 22D-1).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree