Chapter 9 Nerve Disorders and Plantar Heel Pain
CHAPTER CONTENT
A. Lower-extremity nerve injuries in athletes
Introduction
It is my honor to write this chapter for the current edition of the book first edited by Donald E. Baxter, MD, and now edited by David Porter, MD, PhD, and Lew C. Schon, MD. Lew Schon, MD, and Don Baxter, MD, wrote correspondingly the first two authoritative chapters on this subject in 1994.1,2 Much of what they wrote is now among the “classical” knowledge base of foot and ankle surgeons everywhere. Understandably, it is with trepidation that one approaches any changes in “the classics.” Yet, over the past decade, much has been learned related to the pathophysiology, neuroanatomy, and neurodiagnostic testing techniques that can solidify and augment our approach to that base of knowledge related to lower extremity nerve injuries in the athlete.
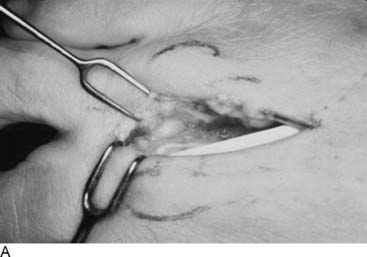
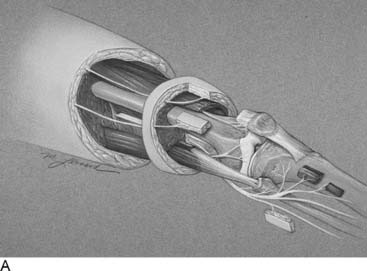
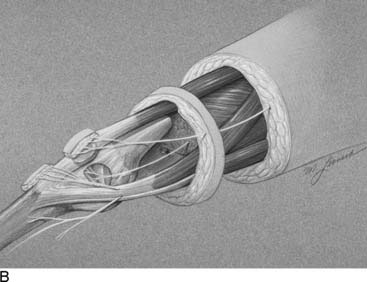
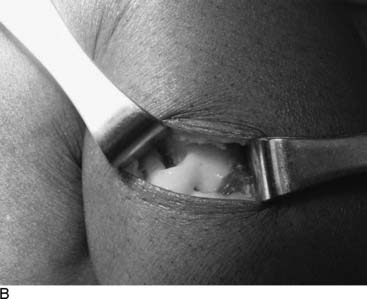
Language is a critical part of our ability to make a diagnosis that relates appropriately to our treatment options. In that regard, today the word “functional” often is used in contrast to “organic,” and therefore I feel it is better to describe peripheral nerve problems related to muscular activity as “exercise-induced” rather than “functional,” because “functional” might connote a psychogenic origin. During diagnostic testing for such an exercise-induced problem, the examiner may use techniques that “provoke” the symptoms by creating compression of the peripheral nerve through certain maneuvers, such as the Phalen sign, wrist flexion test for carpal tunnel syndrome, or maintaining the elbow flexed to provoke the symptoms of cubital tunnel syndrome. Therefore the young athlete who complains of dorsolateral foot pain radiating from the lower leg early in the training process, whether running or dancing, should have an exercise-induced compartment syndrome included in the differential diagnosis, because the basis of that pain is acute nerve compression. Similarly, a “neuroma” is the pathophysiologic process of entrapment of axonal sprouts regenerating into scar tissue.3 A painful neuroma should be treated surgically by resection and implantation of the proximal end into muscle, a technique that has withstood the test of time for the past 20 years for both the upper and lower extremity.4–8 In contrast, chronic compression of a nerve will create an area of narrowing of the nerve with a swelling resulting from axoplasmic accumulation proximal to that point (Fig. 9A-1, A and B). That swelling may appear to be a neuroma but is not a true neuroma. This situation occurs distally in the foot related to the interdigital nerve and the transverse intermetatarsal ligament. The appropriate name for this painful condition should be “interdigital nerve compression” and not “interdigital neuroma.” The history of the origin of this unusual nomenclature has been reviewed recently,9 and the term “interdigital nerve compression in the (given)-interspace,” although cumbersome, is used in this chapter. Resection of a Morton’s neuroma (which is not a true neuroma) results in a true (painful or not) neuroma. Resection is the appropriate surgical treatment for a painful neuroma, and neurolysis is the appropriate surgical treatment for chronic nerve compression.3 The name we ascribe to a given neuropathologic condition therefore has implications for the surgeon.
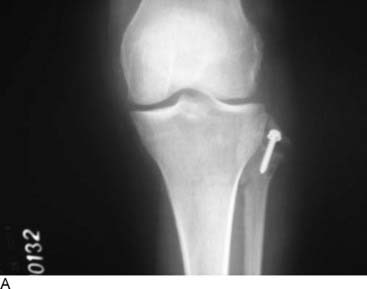
The mechanism of injury results in different degrees of nerve injury. Mechanisms may include direct contusion, stretch/traction, laceration, or acute or chronic compression. The pathophysiology, initially subdivided by Sir Herbert Seddon into three groups (neurapraxia, axonotmesis, neurotmesis), then by Sir Sydney Sunderland into five groups (I-V), and finally by Mackinnon and Dellon into six groups,3 permits diagnosis and prognostication. One could use the knee as an example. The soccer or football player who sustains sufficient force to the medial knee may have a direct contusion to the infrapatellar branch of the saphenous nerve and medial knee pain, which is independent from ligamentous or meniscal structural problems identifiable on a magnetic resonance imaging (MRI). If the infrapatellar branch is simply crushed, recovery of nerve function will be to a normal level, and this will occur within 3 weeks. In contrast, if the force was sufficient to cause disruption of the nerve, bruising, and adherence to the pes anserinus, there will be loss of sensory function and formation of a painful neuroma. If the force of impact is sufficiently great, there may be damage to the meniscus or the collateral ligament, and, in addition to those structural problems, there may be pain resulting from direct damage to the innervation of the knee joint.10 This leads to knee pain of neural origin in addition to the musculoskeletal problems. There may be sufficient lateral force exerted on the knee to cause complete loss of continuity of the common peroneal nerve in addition to the knee joint or fibular injuries (Fig. 9A-2), resulting in peroneal motor and sensory palsy. Therefore “nerve injury” is an insufficient term to specify the neuropathophysiologic cause of the athlete’s pain. Even the term “peroneal nerve palsy” creates ambiguity: Is the correct treatment a period of observation, a neurolysis, or a nerve reconstruction? In this chapter the application of computer-assisted neurosensory testing is included in an attempt to add measurements to the staging of peripheral nerve injury,11 and therefore to the decision-making process.
Joint Pain of Neural Origin
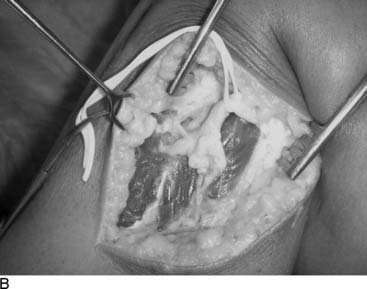
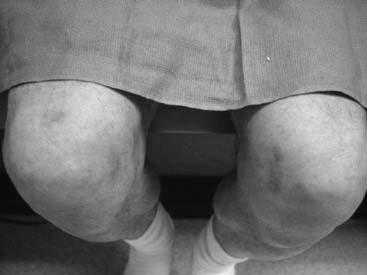
Knee joint pain can be defined to be of neural origin after any musculoskeletal problems with the integrity of the knee joint have been eliminated from the differential diagnosis or have been treated. A common situation is knee pain in the athlete with an MRI that has been interpreted to be normal. Rest, nonsteroidal anti-inflammatory drugs, and intraarticular steroid injections have not been successful at relieving pain. Arthritis may or may not be present radiographically, and if it is, the patient may have failed to improve with treatment designed to form new cartilage. More than likely the patient will have had one or more knee arthroscopies, at which plica has been removed, cartilage has been debrided, or a synovectomy has been performed, but there is still pain. Could this pain be of neural origin? With the description in 1994 of the innervation of the human knee,10 it becomes reasonable to consider that the medial or lateral retinacular nerves, or both, have been stretched or torn and have formed a painful neuroma within the joint structures. Indeed, these nerves may have been injured by the arthroscopy itself. (Figure 9A-3 is an example of a man with bilateral knee pain after years of martial arts, for whom all of the previously mentioned treatments were ineffective and for whom partial knee denervation permitted resumption of daily activities, but not martial arts, without pain. Demonstration of neural origin for the pain is accomplished by (1) local anesthetic block of the suspected nerves, (2) the patient having a decrease in his visual analog scale level of pain by at least 5 points, and (3) observation of improved pain after the block while walking, climbing stairs, and kneeling. If there has been previous knee surgery, a neuroma of a cutaneous nerve, like the infrapatellar branch of the saphenous nerve or the medial cutaneous nerve of the thigh, also must be considered in the examination and the nerve blocks. Figure 9A-4 illustrates the location of these nerves. The first report of partial knee denervation was for patients after total knee arthroplasty,12 but a subsequent report includes patients with knee pain after sports injuries.13 A complete description of this subject appeared in the year 2000.14 The advantage of this approach is that the surgery is performed on an outpatient basis, does not require invasion of the knee joint, and permits immediate ambulation, and rehabilitation can begin when the sutures are removed. Success rates should approach 90% with the previous criteria. Charcot joint does not occur because this is a partial knee joint denervation. The athlete must be counseled that posttraumatic arthritis and synovitis still will occur, but their pain may be significantly lessened.
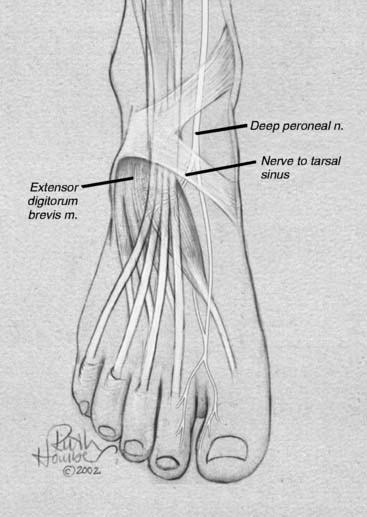
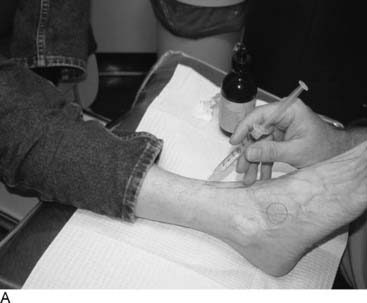
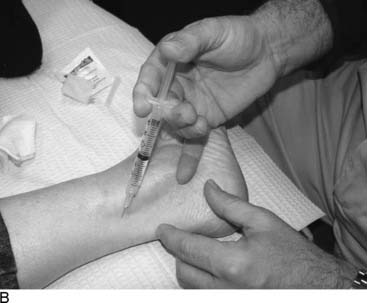
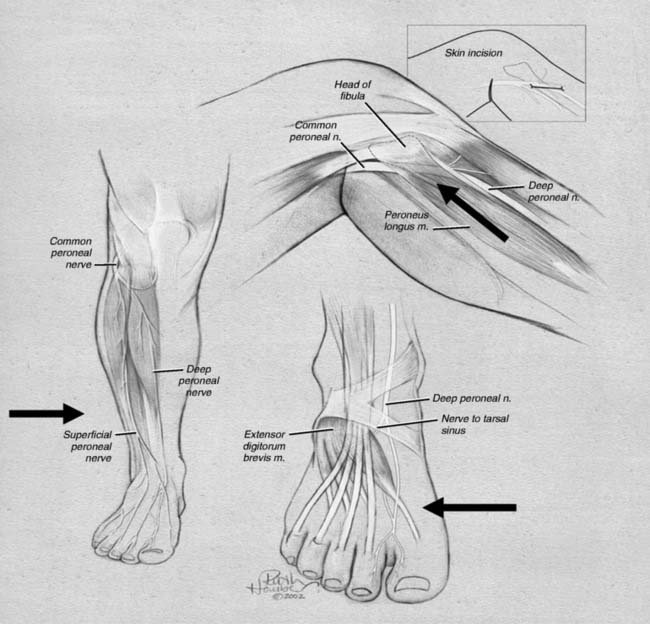
Lateral ankle joint (sinus tarsi) pain can be of neural origin in the athlete who has had repetitive inversion sprains or a fracture/dislocation of the lateral malleolus. Typically, the inversion sprain is treated with immobilization and anti-inflammatories, and then a progressive regimen of stretching and strengthening is begun. If pain persists and x-rays have not demonstrated a fracture, then a computed axial tomography (CAT) scan with three-dimensional reconstruction is suggested to be sure there are not occult intra-articular fractures or bone fragments within the joint as the source of the pain. Any athlete in any sport can suffer this type of injury. If there is a lax lateral ankle joint, surgery to correct it with ligament reconstruction or tendon transfers (Crisman-Snook, or Watson Jones-types) still may have failed to relieve the pain, although providing good stability (see Fig. 9A-12, C). If the symptoms extend to the dorsal lateral aspect of the foot, a stretch/traction injury to the peroneal nerve must be considered in the differential diagnosis, and this is considered later in this chapter. If direct pressure into the sinus tarsi reproduces the pain, a local anesthetic block and steroid injection into this region may be given for both diagnosis and for treatment. If the pain persists, then sinus tarsi syndrome is present. Traditional treatment for this disabling condition has been either a “curettage” (debridement) of the sinus tarsi, resection of the lateral shoulder of the talus, or a subtalar fusion. Could this pain be of neural origin? Recently, the innervation of the sinus tarsi has been demonstrated to come from the deep peroneal nerve.15 The innervation arises most commonly from the most lateral fascicle of the deep peroneal nerve, just proximal to the origin of the innervation of the extensor digitorum brevis in all patients (Fig. 9A-5). About 25% of patients have a dual innervation of the sinus tarsi from the sural nerve.15 With this knowledge, lateral ankle pain can be proven to be of neural origin by blocking the deep peroneal nerve proximal to the ankle. At this site, the nerve is lateral to the extensor hallucis longus and medial to the extensor digitorum longus (Fig. 9A-6, A). If there is not complete relief, then the sural nerve is blocked proximal to the lateral malleolus (Fig. 9A-6, B). The first case of sinus tarsi denervation was reported in 2002 using the technique of resecting just the fascicle that innervates this joint, identifying this by intraoperative nerve stimulation (the most medial fascicle is to the dorsal skin of the first webspace, the central fascicle causes contraction of the extensor brevis muscle on stimulation, and the lateral fascicle is the one innervating the joint).16 In a subsequent series of 13 patients reported in 2005, there were some failures related to this partial resection of the deep peroneal nerve because of joint afferents passing also through the other fascicles.17 The current recommendation therefore is to resect the entire deep peroneal nerve. This is accomplished through an incision located 10 to 12 cm proximal to the lateral malleolus. The anterior compartment is opened with an 8-cm long fasciotomy. The deep peroneal nerve can be identified by dissecting superficial to the interosseous towards the tibia, at which point the nerve is identified with the anterior tibial vessels. A 2-cm length is resected. Immediate weight bearing is permitted. Rehabilitation is begun when the sutures are removed. In these patients, related to the initial stretch/traction injury, there already may be decreased sensibility in the dorsal first webspace and decreased bulk or weakness of the extensor brevis. These should be pointed out to the patient. The numbness present during the block should be pointed out to the patient, who must be informed that this may be permanent after the nerve is resected. The successful relief of pain should approach 90% with this approach. Examples of a patient resuming beach activities and one resuming downhill skiing after this procedure are given in Figure 9A-7.
Peroneal Nerve Injuries
The peroneal nerve arises in the popliteal fossa from the sciatic nerve and travels distally around the fibular neck. There are no sites of entrapment in the popliteal fossa, although the nerve can be injured in this location iatrogenically. Figure 9A-8 illustrates the three most common sites for injury to athletes of this nerve, which consist of sites of anatomic narrowing at which the branches of this nerve are at the risk, namely, the common peroneal nerve at the fibular neck, the superficial peroneal nerve in the distal leg, and the deep peroneal nerve in structures over the dorsal ankle and foot.
The common peroneal nerve can be injured in many athletic activities. The common mechanisms for injury include direct trauma to the knee in contact sports, such as sliding into base in baseball, football, or soccer, and stretch/traction injuries related to inversion sprains of the ankle. Positions such as catcher in baseball put the common peroneal nerve at risk from chronic compression. The most common symptoms include paresthesias into the lateral aspect of the leg and the dorsum of the foot or the perception that the leg is going to “give out.” The first symptom set is related to neural ischemia, which gives rise to the paresthesias in the reversible ischemic block degree of nerve injury. The second set of symptoms, I believe, is related to a similar phenomenon in the motor innervation of the muscles that control ankle dorsiflexion and toe extension. These symptoms are transient and worsen with activity. There may be no positive physical findings with the exception of tenderness of the common peroneal nerve at the neck of the fibula. At this stage of treatment, awareness of this diagnosis is critical, and the treatment is related to relieving pressure on the nerve by changing the athlete’s workout regimen. As symptoms become more persistent, the earliest sensory change will be the pressure required to distinguish one- from two-point static touch using the Pressure-Specified Sensory Device (Fig. 9A-9). Electrodiagnostic testing most likely will still be normal. The first muscle to demonstrate weakness usually is the extensor hallucis longus (see Figure 9A-9). With the onset of muscle weakness or the increase in distance required for two-point discrimination, the degree of compression is sufficiently chronic and severe to justify neurolysis of the common peroneal nerve. This is illustrated in Figure 9A-10. The incision is oblique at the fibular neck. Care is taken not to injure the occasionally present lateral cutaneous nerve of the calf. The fascia is opened and the common peroneal nerve identified. The nerve is followed distally to the entrapment site at the peroneus longus muscle. If there has been direct knee trauma, the fascia will be adherent to the nerve, and the neurolysis then is continued proximally into the popliteal fossa. Recent observations have made the following modifications to the procedure as previously described18: 20% of cadavers but 80% of patients will have a fibrous band deep to the peroneus longus muscle. This must be searched for and divided if present, and there often will be a notch at this location in the nerve (Fig. 9A-11). A smaller percentage of patients also will have fibrous bands on the surface of the lateral head of the gastrocnemius muscle, deep to the nerve, and these must be released. Finally, a high origin of the soleus muscle may narrow the entrance of the nerve into the anterolateral compartment, and this origin must be released from the fibula.19
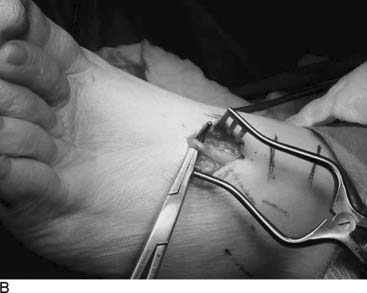
The superficial peroneal nerve has a variable anatomy in the leg. Up to 40% of cadavers have the superficial peroneal nerve either completely in the anterior compartment (Fig. 9A-12) or a branch in the anterior and a branch in the lateral compartment (Fig. 9A-13).20,21 The superficial peroneal nerve exits from beneath the deep fascia to become subcutaneous in the distal third of the leg. This site is quite variable but is most commonly located 10 to 12 cm proximal to the lateral malleolus (see Fig. 9A-8). In young athletes, such as runners or dancers, this site can be compressed because the muscles of the anterior and lateral compartment bulge during exercise, creating ischemic neuralgia. This pain goes away with cessation of the physical activity. The diagnosis should be made with compartment pressure measurements while the patient is in a controlled exercise environment, such as on a treadmill. Elevation of pressure to greater than 30 mm Hg coinciding with the pain is an indication for fasciotomy. Although the fasciotomy incision does not need to be long, the fasciotomy should be extensive to prevent a small muscle herniation, which itself can be painful. The fascia is well vascularized, and its edges should be cauterized to minimize postoperative bruising or hematoma. Care is taken to not injure the nerve, and the neurolysis must be extended proximally until the nerve is surrounded by muscle and distally until the nerve enters the subcutaneous tissue (see Fig. 9A-12). For soccer players, this nerve can be injured directly by the ball or by being kicked. Similar injuries can occur in field hockey and lacrosse. Symptoms of chronic compression of the superficial peroneal nerve are paresthesias from the distal leg into the top of the foot, without motor symptoms. There will be a positive Tinel sign at the site of compression. Sensory testing will demonstrate normal sensibility in the dorsal first webspace and abnormal sensibility over the dorsolateral foot. Results of neurolysis of this nerve are good to excellent in 85% of patients.21,22 Surgery is performed on an outpatient basis, and immediate ambulation is encouraged to permit gliding of the nerve through the surgical site. Rehabilitation begins when the sutures are removed.
Figure 9A-13 Intraoperative view of neurolysis of superficial peroneal nerve. In this patient, there was a branch of the nerve in both the anterior and the lateral compartment, emphasizing, as in Figure 9A-12, the need to release both compartments.
The deep peroneal nerve has been described classically as being entrapped beneath the extensor retinaculum in front of the ankle, with this location being called the anterior tarsal tunnel, and the clinical symptoms being termed the anterior tarsal tunnel syndrome.23–25 In my experience, this is a capacious region, with the deep peroneal nerve between tendons, bone, extensor retinaculum, and fat and little chance for compression unless there has been a crush, a burn, an ankle fusion, or previous surgery (see Fig. 9A-8). In contrast, just distal to the inferior crus of the extensor retinaculum the extensor hallucis longus tendon crosses the deep peroneal nerve in close proximity to the base of the first metatarsal and the cuneiform. This is the area in which ganglions arise, and a dorsal exostosis forms. This exact site was described as a location of chronic nerve compression in 1990.26 The deep peroneal nerve at this location innervates the joints of the first and second metatarsals and the cuneiform bones, and therefore symptoms of pain from the dorsum of the foot through to the plantar surface, like a “knife stabbing” this location, can occur, in addition to the aching in the forefoot and paresthesia in the webspace. This is a site that has been illustrated by Schon2 to be a risk for ballet dancers in the pointe position and can be directly injured and the source of persistent pain following metatarsal stress fractures or Lisfranc fracture/dislocations. Tightly fitting athletic shoes have been reported to cause compression of the deep peroneal nerve,23–25 including by ski boots. 27 I have seen this to be the problem in an ice skater who had pain in this area on the take-off and landing of her spins. Loosening the laces on her skates did not help. There was a positive Tinel sign at this location. Radiographic evaluation, including MRI, had been normal. Her electrodiagnostic studies had been normal (with the more proximal anterior tarsal tunnel location, electromyogram [EMG] of the extensor brevis digitorum brevis muscle can help in the diagnosis), but her neurosensory testing with the Pressure-Specified Sensory Device demonstrated increased pressure threshold and abnormal two-point static threshold for distance. At surgery, her deep peroneal nerve clearly was indented by the extensor hallucis brevis tendon (see Fig. 9A-1, A), and this tendon was removed. She was able to resume ice skating at 10 days and return to doing jumps and spins without pain.
Posterior Tibial Nerve Injuries
The proximal posterior tibial nerve arises from the sciatic nerve in the popliteal fossa, and there is no anatomic site of entrapment in that location, although the nerve can be injured iatrogenically here. For example, a 21-year-old college student injured her knee skiing. During her arthroscopy, the scope perforated the posterior capsule of the knee and avulsed the popliteal artery and vein and the posterior tibial nerve. After her emergent vascular reconstruction, she was referred at 3 months for nerve reconstruction. This required direct neurotization of the gastrocnemius muscles because their motor branches were avulsed, and nerve grafting to provide distal sensation to the plantar aspect of her foot. By 2 years after this reconstruction, she had active plantarflexion of the ankle and protective sensation to the plantar aspect of her foot.28 The posterior tibial nerve continues distally to enter the distal leg by passing between a fibrous arcade formed between the two heads of the gastrocnemius and the soleus muscle. Theoretically, this can be a site of compression, and decompression at this level has been described by Baxter1 as the “high tarsal tunnel.” I have had occasion to decompress the nerve in this region in two patients who had chronic compartment syndrome, and one of these was related to a sports injury. That patient developed an acute compartment syndrome related to a tight ski boot, which went without a proper diagnosis. When the leg was decompressed, about 48 hours later, the fasciotomy was proximal and inadequate. Muscle was debrided, and the wound skin grafted secondarily. By the time the patient was referred to me 3 years later, there was a chronic compartment syndrome associated with severe pain from the knee to the toes. There was inability to flex or extend the toes or ankle. The patient required a wheelchair and was under pain management, requiring a Duragesic patch, methadone, Neurontin, and fentanyl lollipops. The pain was relieved and all motor function recovered by carrying out a neurolysis of the tibial nerve in the calf, along with a four-compartment fasciotomy and a neurolysis of the common peroneal nerve at the knee. The superficial peroneal nerve had been divided during the original fasciotomy, requiring for the neuroma now to be resected and implanted into muscle (Fig. 9A-14). However, the term “tarsal tunnel” should be reserved for a compression site in relation to the tarsal bones and not in the midcalf.
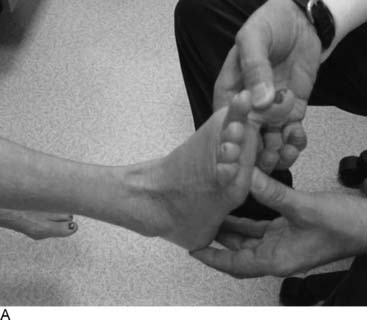
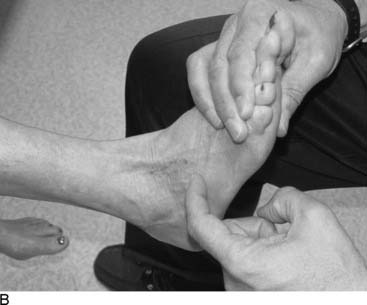
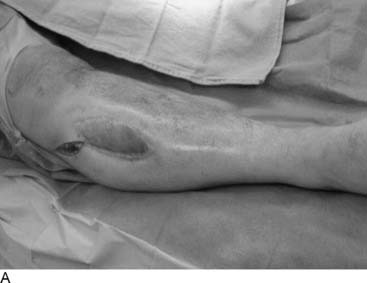
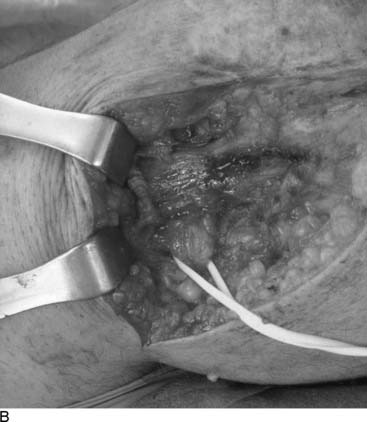
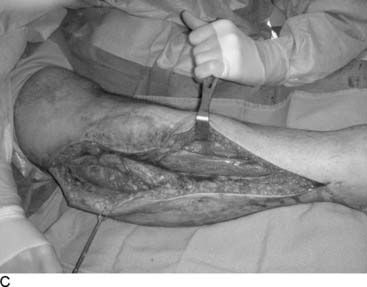
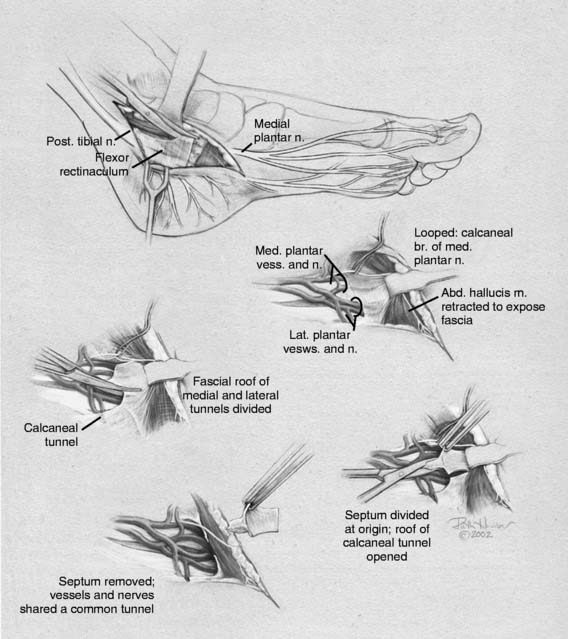
Tarsal tunnel syndrome was described in 1962 but remains relatively misunderstood. It is often referred to as the “carpal tunnel syndrome of the foot.” When my first patient was referred to me in about 1980, and I studied this statement, I realized that in fact the tarsal tunnel region was analogous to the forearm and not the carpal tunnel. Subsequently the appropriate relationships were published and are given in Table 9A-1.29 The patient with carpal tunnel syndrome would not be relieved by a forearm fasciotomy, and so an operation was designed to decompress the four medial ankle tunnels (Fig. 9A-15). It is not appropriate to call this operation “tarsal tunnel decompression” alone because four separate tunnels are decompressed. It is confusing to speak of the tarsal tunnel as the “upper tarsal tunnel” and the medial and lateral plantar tunnels as the “distal” or “lower tarsal tunnel.” In this chapter, each tunnel is called by its correct anatomic name. Compression of the nerves in the four medial ankle tunnels can occur in an athlete through several different mechanisms. The medial ankle may be injured directly. Inversion sprains may create sufficient bruising and swelling so that the posterior tibial nerve and its branches become adherent during the immobilization of the ankle for 3 weeks, subsequently giving rise to chronic compression. Repetitive trauma can occur in runners, cyclers, those who do step aerobics, and so forth. The pronated foot theoretically is more likely to have pressure applied to the posterior tibial nerve branches, although this remains to be proven. A fracture/dislocation of the ankle or severe inversion sprain may directly contuse the tibial nerve and its branches. Swelling or blood products related to posterior tibial nerve tears or avulsion of a portion of the navicular may cause compression of the medial plantar nerve. Symptoms of tarsal tunnel syndrome include aching, paresthesias, or numbness in the heel, arch, forefoot, or toes. Nighttime discomfort is common. In time, muscle weakness and clawing of the intrinsic muscles occur (Fig. 9A-16, A) and the paresthesias progress to constant numbness. Electrodiagnostic testing can document this problem but has a high false-negative rate. Neurosensory testing with the Pressure-Specified Sensory Device (NK Biotechnical, Minneapolis, MN) can document this problem at an earlier stage than electrodiagnostic testing.30–32 An example of the documentation of tarsal tunnel syndrome with neurosensory testing is given in Figure 9A-16, B. Rehabilitation after decompression of the four medial ankle tunnels is critical to the success of the surgery. It should be clear that if a nerve is permitted to remain immobilized during the wound healing process, the formation of collagen in that process will result in scarring of the nerve into the surgical field, causing failure of the original neurolysis.34 Early if not immediate mobilization of the nerve must be part of the postoperative regimen for the tibial nerve at the ankle as it is for the ulnar nerve at the elbow.35 Although there are many reviews of tarsal tunnel syndrome, for example the one by Lau and Daniels,36 there are no reported series larger than 68 patients, and their reported surgical results vary considerably. For example, an often-quoted study, in which long-term follow-up was obtained, reported just 44% of the patients with excellent outcomes and a 13% complication rate,37 whereas a more recent report indicated 72% of the patients with satisfactory results but a 30% complication rate.38 The most recent report, using outcome assessment, found 51% of the patients having a marked improvement in the quality or their life despite 85% of the patients stating they had excellent relief of their pain, with a 7% rate of complications.39 Using the technique I described previously, a consecutive series of 87 legs in 77 patients had the four medial ankle tunnels decompressed between January of 1987 and December of 1994. The follow-up was a mean of 3.6 years. The results were 82% excellent, 11% good, 5% fair, and 2% failure.40 Using a numerical grading scale,41 there was a statistically significant improvement at the p < .001 level for each preoperative grade of impairment with the exception of level 10, intrinsic contracture. My experience has grown considerably in posterior tibial nerve decompression, with more than 600 procedures being listed in my computer between January 2000 and June 2005. This large experience is due, in part, to the application of this technique to relief of pain and restoration of sensation to patients with neuropathy resulting from diabetes,42–44 chemotherapy,45 or idiopathic causes.46 None of the patients have required repeat surgery with the previously mentioned operative and postoperative regimen. Results of an ongoing, multicenter prospective study are available online at www.neuropathyregistry.com.
Table 9A-1 Relationship between the four medial ankle tunnels and the nerve compression sites in the upper extremity
| Sites of nerve compression | |
|---|---|
| Lower extremity | Upper extremity |
| Tarsal tunnel | Forearm |
| Medial plantar tunnel | Carpal tunnel |
| Lateral plantar tunnel | Guyon’s canal |
| Calcaneal tunnel | Palmar cutaneous branch of median nerve tunnel33 |
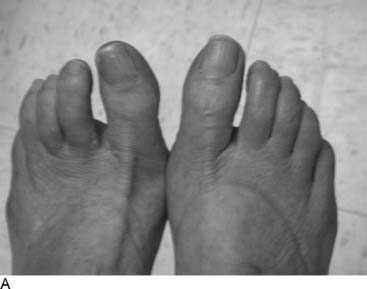
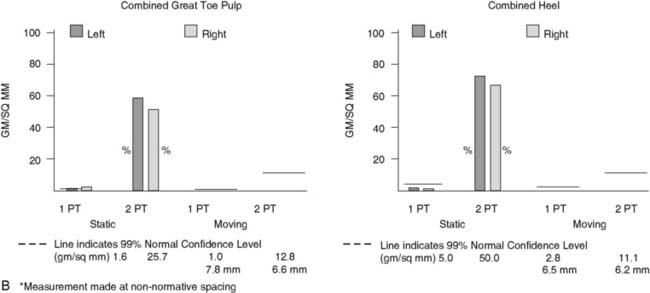
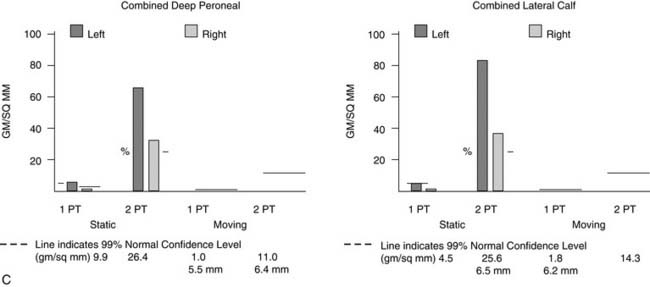
Figure 9A-16 Physical examination and neurosensory testing related to posterior tibial nerve compression. (A) Clawing, a sign of lateral plantar nerve compression, often is mistaken for hammertoes. In this patient with bilateral severe tarsal tunnel syndrome related to neuropathy, decompression of the four medial ankle tunnels on the right side has permitted reversal of the clawing on one side, while it remains on the other, nonoperated side. (B) Evaluation of the sensory component with the Pressure-Specified Sensory Device results in a computer printout in which the pressure required to discriminate one- from two-point static touch is measured. In this report the left, blue bars are above the 99% confidence limit for pressure for age (the horizontal black bar), indicating abnormal function for the left side, whereas the right side, in red, is below the bar. The asterisk denotes abnormal distance for the measurement, indicating axonal loss. The sensibility is abnormal for both the medial plantar nerve (hallux pulp) and the medial calcaneal nerve (heel, medial surface), indicating that both of these nerves are abnormal and that the location of the compression is in both the medial plantar tunnel and the calcaneal tunnel. The surgical approach is given in Figure 9A-15.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree