Amputation Surgeries for the Lower Limb
Michelle M. Lusardi and Judith L. Pepe
Learning objectives
On completion of this chapter, the reader will be able to do the following:
When is amputation necessary?
The amputation of a limb is a truly life-altering event, affecting the physical, functional, and psychological dimensions of a person’s world.1 The decision to amputate a limb is not made lightly; amputation is performed as a lifesaving measure for those affected by severe trauma,2 infection,3 peripheral arterial disease,4 and certain cases of osteosarcoma.5 Amputation can be incredibly life improving when burdensome care of a vulnerable neuropathic or painful dysvascular foot is no longer necessary.6 Unexpected traumatic amputations, especially in younger active individuals, can contribute to significant depression in the postoperative and rehabilitation periods.7
Although surgical technique is one of the key determinants in the success of amputation,8 other factors that are as important include the individual’s and family’s understanding and acceptance that amputation is the best option of care9; effective attention to and management of comorbidities during the perioperative period, as well as avoidance of postoperative complications such as pneumonia and wound infection10,11; and a promptly implemented rehabilitation plan that the individual, family, surgical team, and rehabilitation team have developed and agreed on.12
This chapter provides an overview of the strategies used to determine if amputation is necessary for individuals with dysvascular/neuropathic disease, for those who have sustained significant trauma to their lower limbs, for those with diagnoses of bone or soft-tissue neoplasm, and for children who are growing up with congenital limb deficiency. We then describe the surgical techniques used in the most commonly performed lower-extremity amputation and discuss the rehabilitation implications for each. The goal is to help rehabilitation professionals understand what has happened to soft tissue and bone during surgery and to use this knowledge to better incorporate principles of wound healing and maturation into the preprosthetic and prosthetic care of those who have undergone amputation.
Basic Principles
The decision to amputate a limb, whether related to critical limb ischemia, infection of a neuropathic wound, trauma, or neoplasm, is based on careful consideration of prognosis for survival and for successful rehabilitation. Determination of the appropriate level of amputation is guided by two principles. The first is that there must be adequate circulation to ensure successful healing of the incision and surgical construct.13 In addition to a physical examination (evaluation of peripheral pulses, skin color, skin condition, and skin temperature), surgeons consider the individual’s ankle-brachial index,14 results of transcutaneous oximetry,15 and vascular studies such as magnetic resonance arteriography.16 The second principle is to preserve as many anatomical joints as possible; having an intact anatomical knee joint typically leads to better functional outcome.14
At times, tension occurs between these principles. For individuals with plantar neuropathic ulcers and osteomyelitis, for example, a transmetatarsal amputation has the potential to preserve the ankle joint and permit functional gait without prosthesis and is often less difficult for individuals to accept psychologically.17 If there is delayed or failed healing, however, the risks of complications associated with limited activity and bed rest (e.g., further deconditioning, pneumonia, deep venous thrombosis, decubitus ulcer) and repeated anesthesia if surgical revisions to more proximal levels become necessary can be significant.18 In these instances an initial surgery at the transtibial level might improve the chances for optimal rehabilitation outcome.19
In individuals with traumatic crush injury to the proximal tibia but intact knee joint, an extremely short transtibial residual limb may actually be more difficult to manage prosthetically than a long transfemoral residual limb: the reduced surface area around a short residual tibia and fibula for weight bearing within the socket increases pressure on skin and soft tissue of the residual limb, reducing functional wearing time of the prosthesis despite the advantage of preservation of the anatomical knee joint.20
Dysvascular and Neuropathic Disease
The overall prevalence of peripheral arterial disease (PAD) in the United States, across all ethnicities, is estimated to range from approximately 2% in persons 40 to 49 years of age to 20% or higher in persons older than age 80 years.21 Rates of PAD vary by ethnicity and gender, however; African-American men demonstrate two- to threefold greater rates than white men, whereas women demonstrate lower rates than men of their ethnicity (Table 19-1).21,22 Likelihood of amputation increases with the number of risk factors for PAD an individual has. Risk factors include history of smoking (especially if currently smoking); untreated or poorly managed hypercholesteremia; untreated or poorly managed hypertension, obesity (body mass index >30); diabetes mellitus, kidney dysfunction (glomerular filtration rate <60 mL/min), and chronic inflammatory response (elevated high-sensitivity C-reactive protein >30 mg/L).23 Significant morbidity and mortality is associated with PAD: It is estimated that one in four individuals with PAD undergoes some form of amputation, one in three will likely die within 5 years of diagnosis, and only one in four will survive more than 10 years after diagnosis.24,25 Comorbid conditions that amplify risk of death in the year following PAD-related amputation include congestive heart failure, renal failure, and liver disease, as well as postoperative systemic sepsis.26 PAD is a significant health care issue, impacting not only on the quality of an individual’s life, but also on ever-increasing health care costs.27,28
Table 19-1
Prevalence of Peripheral Arterial Disease by Age and Gender
| Age Category, % (CI) | ||||||
| Gender | Ethnicity | 40-49 | 50-59 | 60-69 | 70-79 | ≥ 80 |
| Male | NHW | 1.4 (0.3-2.5) | 1.9 (0.9-2.9) | 5.4 (3.9-6.9) | 9.2 (7.9-10.5) | 22.6 (18.6-26.3) |
| AA | 1.2 (0.0-2.5) | 5.0 (2.6-7.3) | 13.2 (10.0-16.3) | 24.4 (20.3-28.6) | 59.0 (45.6-68.6) | |
| HS | 0.2 (−0.4-0.8) | 3.4 (1.3-5.4) | 4.3 (2.1-6.5) | 9.6 (6.0-13.2) | 22.5 (9.4-34.5) | |
| AS | 1.2 (−1.1-3.5) | 0.9 (−0.6-2.3) | 3.5 (0.7-6.4) | 9.8 (8.7-11.0) | 21.5 (18.8-24.0) | |
| AI | 2.6 (1.3-4.0) | 4.5 (2.9-6.1) | 6.1 (3.8-8.4) | 11.7 (5.5-17.8) | 28.7 (24.2-32.6)* | |
| Female | NHW | 1.9 (0.7-3.2) | 4.3 (2.9-5.7) | 5.1 (3.7-6.4) | 7.9 (6.9-9.0) | 18.2 (15.2-21.2) |
| AA | 3.0 (1.2-4.7) | 3.4 (1.7-5.1) | 8.9 (6.6-11.1) | 20.0 (16.8-23.2) | 35.1 (27.0-43.2) | |
| HS | 0.3 (−0.3-1.0) | 0.4 (−0.2-3.0) | 3.1 (1.3-4.9) | 6.9 (3.7-10.2) | 18.2 (7.6-28.8) | |
| AS | 0.0 (0.0-0.0) | 1.4 (−0.2-3.0) | 0.7 (−0.5-2.0) | 7.9 (3.4-12.4) | 18.2 (3.1-33.3)* | |
| AI | 3.2 (1.9-4.5) | 3.9 (2.7-5.1) | 8.6 (6.4-10.8) | 14.7 (9.5-19.9) | 33.8 (30.1-37.4)* | |
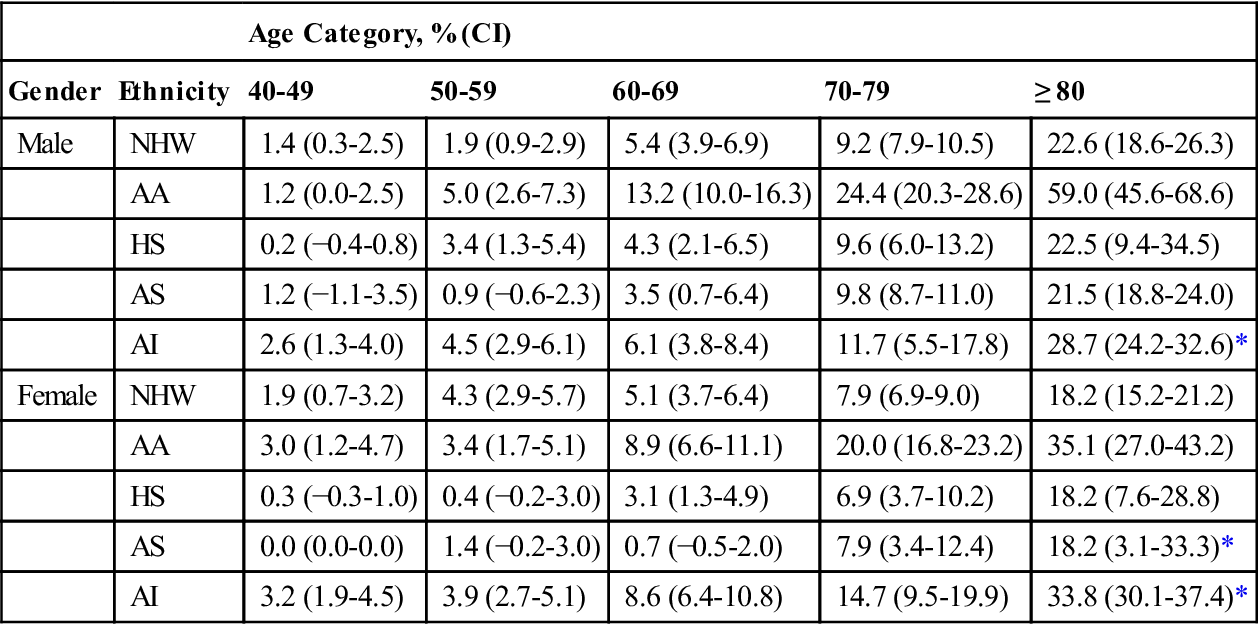
AA, African-American; AI, American Indian; AS, Asian American; CI, confidence interval; HS, Hispanic, NHW, non-Hispanic white; OTH, other.
Results are presented as prevalence estimates (95% CI).
From Allison MA, Ho E, Denenberg JO, et al. Ethnic-specific prevalence of peripheral arterial disease in the United States. Am J Prev Med. 2007;32(4):328-333; Table 2, p. 330.
There are three reasons an individual might require revascularization or amputation of the lower limb because of underlying disease processes: (1) they have significant, often acute, occlusive atherosclerotic vascular disease but without neuropathy or diabetes; (2) they are without significant vascular disease but have neuropathy-related wounds that may lead to osteomyelitis; and (3) they have diabetes with a combination of vascular disease and sensory, motor, and autonomic neuropathy.29,30 The management and prognosis for each of these groups are somewhat different. Vascular bypass surgery,31 percutaneous endovascular stents,32 or the use of thrombolytic intervention33 may preserve the limbs of those with large-vessel vascular disease without neuropathy. Recent reports suggest that intramuscular injection of autologous bone marrow cells may decrease risk of amputation in persons with chronic limb ischemia who are not candidates for surgical revascularization, or when such surgeries are unsuccessful.34,35 Persons with a combination of diabetes and vascular disease are the most likely to require amputation; advanced age and multiple comorbidities (e.g., cardiovascular and cerebrovascular disease, kidney disease, and visual impairment) provide additional challenges for healing, early mobility after amputation, and the prosthetic rehabilitation process.
Conservative management with total contact casting or felted foam may be sufficient to heal a neuropathic ulcer (when there are no indications of osteomyelitis), and accommodative orthoses and protective footwear may protect the plantar surface of the foot in those with a healing neuropathic wound.36 Hyperbaric oxygen therapy and negative-pressure wound therapy also have been investigated as strategies to close neuropathic wounds and reduce risk of limb amputation in persons with chronic limb ischemia and diabetes.37,38 When neuropathic or vascular foot wounds become infected or osteomyelitis is evident, conservative management can be challenging, and amputation may be necessary.39
Because both vascular disease and neuropathic disease are typically symmetrical in distribution, individuals in all of these groups are at risk for compromise of both lower limbs. After amputation of one limb, careful monitoring of vascular status and skin condition and appropriate conservative care of the intact limb and foot are essential. This is especially true in the postoperative–preprosthetic period when there is single-limb ambulation with assistive devices, as well as in the months and years following initial amputation.40,41
For all of these individuals, clinical decision making must be informed by careful assessment of (a) vascular status to determine whether revascularization or amputation is warranted, (b) cardiovascular and cardiorespiratory function to determine the most appropriate type of anesthesia to use during surgery, and (c) cognitive and psychological status to assure proper perioperative and postoperative care and patient education.
The assessment of an individual with compromised peripheral circulation begins with a careful and detailed health history and review of risk factors, continues with physical examination and routine blood work, and is followed up by additional tests or measures as needed.42,43 Cognitive and psychological status is initially assessed by interview and as part of the neurological examination and can be followed up by more formal testing if warranted. Vascular status is initially examined using noninvasive methods such as the ankle-brachial index (ABI); values less than 0.9 suggest PAD.44 Any suspicion of vascular compromise should be further investigated using duplex ultrasound,45 magnetic resonance arteriography (MRA),46 or computed tomographic arteriography (CTA). The physician’s differential diagnosis process focuses on determining which component of the circulatory system is involved (arterial, venous, or lymphatic), if it is an acute problem that requires immediate medical, pharmacological or surgical intervention (e.g., vascular bypass or amputation), or a chronic problem that necessitates conservative medical management (Table 19-2).
Table 19-2
Common Peripheral Vascular Disorders
| System | Acute Conditions | Chronic Conditions |
| Arterial | Arterial thrombosis Embolic occlusion Vasospastic disease (Raynaud) | Atherosclerosis obliterans Thromboangiitis obliterans Buerger disease Diabetic angiopathy |
| Venous | Venous thromboembolism | Varicose veins Chronic venous insufficiency |
| Lymphatic | Lymphangiitis | Primary lymphedema (congenital) Secondary lymphedema (acquired) |
Vascular Pain
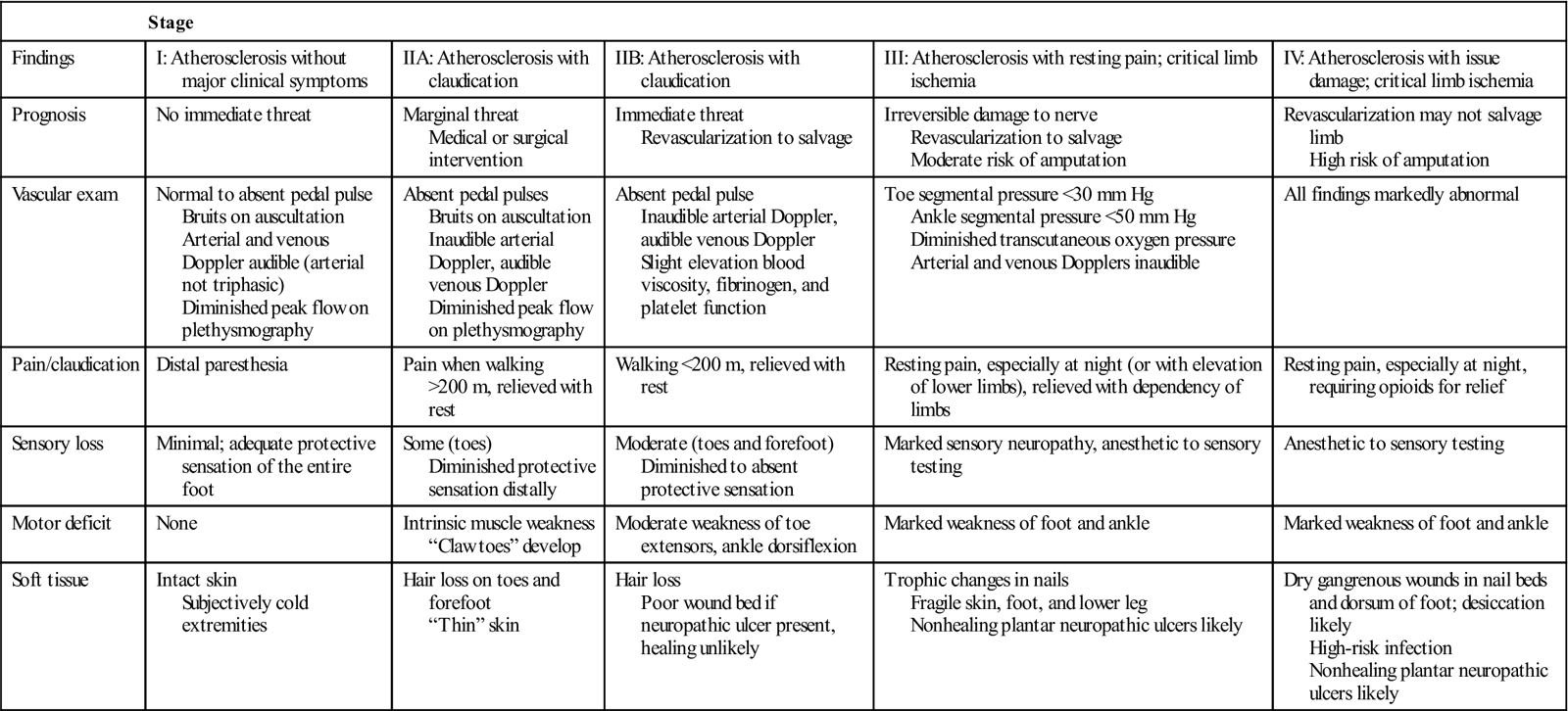
One of the most common symptoms of chronic arterial vascular insufficiency is intermittent claudication.47 This vascular-related pain has been described as a deep aching, cramping, muscle fatigue, or tightness that develops during physical activity and dissipates with rest. Although most common in the posterior compartment (gastrocnemius and soleus) of the lower leg, claudication can occur in any muscle with compromised blood supply, including the muscles of the thigh and hip. Claudication is the result of accumulation of lactic acid as a byproduct of anaerobic metabolism during muscle contraction. Several strategies are used to classify severity of PAD and of intermittent claudication. In 1954, Fontaine first defined four stages of PAD on the basis of the presence and severity of clinical symptoms (Table 19-3).48 In 2007, the Trans-Atlantic Inter-Societal Consensus Task Force published a classification system (TASC II) for intermittent claudication with recommendations for appropriate intervention (endovascular angioplasty with or without stent placement, versus open surgical endarterectomy or bypass graft) based on location, length, and severity of occlusion or stenosis obtained from imaging studies.49 Severity of PAD can be quantified using treadmill testing at a constant 2 mph speed, beginning flat and continuing with 2% increase in grade every 2 minutes, noting time to onset of claudication and time to maximum claudication. Change in the total distance that an individual is able to walk (e.g., number of city blocks or actual measured distance) before onset of symptoms is an alternative means of tracking progression of arterial vascular impairment.50 When an individual requires opiates to manage vascular pain while at rest, or if there are arterial ulcers with dry gangrene in the toes or foot, the individual is classified as having critical limb ischemia, and is at high risk of amputation if revascularization fails or cannot be undertaken.51
Table 19-3
| Stage | |||||
| Findings | I: Atherosclerosis without major clinical symptoms | IIA: Atherosclerosis with claudication | IIB: Atherosclerosis with claudication | III: Atherosclerosis with resting pain; critical limb ischemia | IV: Atherosclerosis with issue damage; critical limb ischemia |
| Prognosis | No immediate threat | Marginal threat Medical or surgical intervention | Immediate threat Revascularization to salvage | Irreversible damage to nerve Revascularization to salvage Moderate risk of amputation | Revascularization may not salvage limb High risk of amputation |
| Vascular exam | Normal to absent pedal pulse Bruits on auscultation Arterial and venous Doppler audible (arterial not triphasic) Diminished peak flow on plethysmography | Absent pedal pulses Bruits on auscultation Inaudible arterial Doppler, audible venous Doppler Diminished peak flow on plethysmography | Absent pedal pulse Inaudible arterial Doppler, audible venous Doppler Slight elevation blood viscosity, fibrinogen, and platelet function | Toe segmental pressure <30 mm Hg Ankle segmental pressure <50 mm Hg Diminished transcutaneous oxygen pressure Arterial and venous Dopplers inaudible | All findings markedly abnormal |
| Pain/claudication | Distal paresthesia | Pain when walking >200 m, relieved with rest | Walking <200 m, relieved with rest | Resting pain, especially at night (or with elevation of lower limbs), relieved with dependency of limbs | Resting pain, especially at night, requiring opioids for relief |
| Sensory loss | Minimal; adequate protective sensation of the entire foot | Some (toes) Diminished protective sensation distally | Moderate (toes and forefoot) Diminished to absent protective sensation | Marked sensory neuropathy, anesthetic to sensory testing | Anesthetic to sensory testing |
| Motor deficit | None | Intrinsic muscle weakness “Claw toes” develop | Moderate weakness of toe extensors, ankle dorsiflexion | Marked weakness of foot and ankle | Marked weakness of foot and ankle |
| Soft tissue | Intact skin Subjectively cold extremities | Hair loss on toes and forefoot “Thin” skin | Hair loss Poor wound bed if neuropathic ulcer present, healing unlikely | Trophic changes in nails Fragile skin, foot, and lower leg Nonhealing plantar neuropathic ulcers likely | Dry gangrenous wounds in nail beds and dorsum of foot; desiccation likely High-risk infection Nonhealing plantar neuropathic ulcers likely |
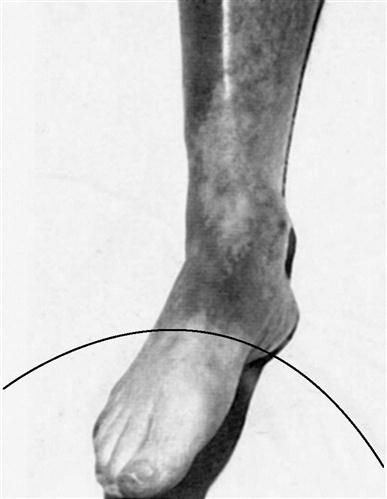
When there is an acute or sudden occlusion of an arterial vessel (such that collateral circulation has not had sufficient time to develop), a much different pattern of pain occurs. Instead of relief with rest, pain is constant and somewhat unrelenting; it may be accompanied by feelings of tingling, numbness, or coldness as peripheral nerves of the lower limb are affected by ischemia.34 Although a limb with chronic arterial insufficiency demonstrates dependent rubor, the skin of an acutely compromised limb may be quite pale or blanched distal to the site of occlusion (Figure 19-1). Acute occlusion is often an emergent situation, requiring pharmacological or surgical intervention to restore blood flow to the limb, and often necessitates long-term anticoagulation.
Examination: Skin and Soft Tissue
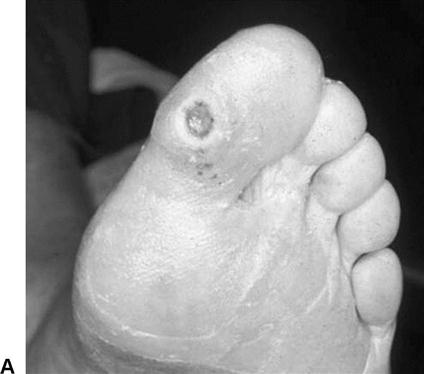
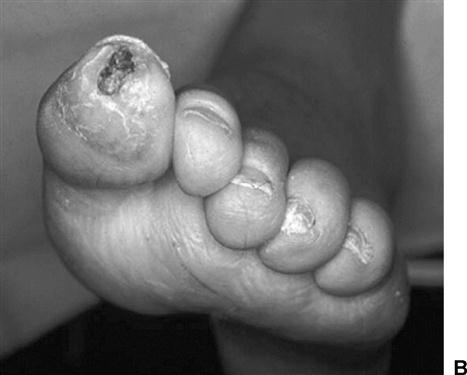
Assessment begins with visual inspection of the lower extremity and feet, concentrating on skin condition, presence or absence of hair, and nail condition.52 Open wounds, callus, ecchymosis, dry necrosis and other skin lesions, as well as areas of erythema (redness), mottling, and altered pigmentation, are documented on a body chart. Circular wounds that lie under or are surrounded by callus on the plantar or weight-bearing surfaces of the foot are most likely neuropathic ulcers (Figure 19-2, A). Dry, blackened, or moist gangrenous wounds in the nail beds and between toes are likely signs of vascular insufficiency (Figure 19-2, B). Although “healthy” traumatic wounds often display clear serosanguineous drainage, any thickened, yellowish, or foul-smelling discharge from a wound suggests soft tissue or bone infection.
Open wounds around ankles or a distal leg surrounded by areas of darkly pigmented skin are typically the result of chronic venous stasis. Progressive circumferential measurements are taken to document the presence of pitting or nonpitting edema resulting from venous or lymphatic insufficiency, localized infection, or as part of an underlying systemic disease of kidney, heart, or lung. Skin temperature is assessed subjectively (using the web space or back of the examiner’s hand) or objectively using a surface thermometer. Cold areas in particular may have compromised arterial flow, while areas of increased temperature (especially if accompanied by erythema and swelling) often indicate underlying infection.52
In the presence of diabetic and other polyneuropathies there is often atrophy or wasting of the intrinsic muscles of the foot with apparent claw or hammer toe deformity.53 Protective sensation (as measured by perception of Semmes-Weinstein 5.07 filament), as well as touch, pressure, vibration, and position sense, are likely to be impaired or inconsistent.54 Additionally, impairment of the autonomic system often causes dry, tight, shining, easily cracked skin, as well as altered dynamics of blood flowing to the bones of the foot, increasing risk of Charcot arthopathy.55,56
Vascular Examination: Noninvasive Strategies
The least invasive and simplest strategy used to assess adequacy of vascular supply to the distal limb is palpation of distal lower-extremity pulses at the dorsalis pedis and posterior tibial arteries, popliteal pulse at the knee, and femoral pulse at the groin. Strength of pulses at each site is noted as absent (0/4), weak (1/4), normal (2/4), full (3/4), or bounding (4/4).40 If all pulses are palpable, it is likely that there is adequate circulation to heal a neuropathic ulcer. Absent distal pedal pulses do not, however, confirm vascular insufficiency; it is estimated that pedal pulses are nonpalpable in up to 10% of the general population.57
When pedal pulses are not palpable, further assessment might include positional tests of capillary and venous filling time, auscultation with Doppler ultrasound, calculation of an ABI, taking segmental blood pressure of the limb, transcutaneous oximetry (pulse oximetry), pulse-volume recordings, MRA, computed tomography (CT) angiography, or duplex scanning.58 (Refer to Chapter 18 for a detailed description of noninvasive assessment strategies.) Table 19-4 lists reference ranges for noninvasive tests.
Table 19-4
Reference Values for Noninvasive Examination of Peripheral Arterial Circulation
| Test/Measure | Normal Values | Abnormal Findings |
| Capillary refill time | Elevation of limb 20 seconds Return to dependent position Pressure on toe or nail Blanch, then refill in 1 to 2 seconds | Delayed refill or persistent blanching Rubor of dependency |
| Refill after occlusion | Inflation of blood pressure cuff at thigh for 5 minutes On release, flush to normal skin color at toes within 10 seconds | >10 seconds: impaired arterial perfusion |
| Venous refill time | Elevation of the limb 2 minutes Return to dependent position Veins on dorsum of foot refill in 10 seconds | >10 seconds: impaired arterial perfusion <10 seconds: valvular incompetence of veins |
| Doppler ultrasound | Triphasic on auscultation | Biphasic: mild vascular impairment Monophasic: significant impairment Absent: complete occlusion |
| Ankle-brachial index | 0.9 to 1 | <0.9 impaired arterial flow <0.5 unlikely to heal distal wound |
| Segmental blood pressure | <15 mm Hg drop in systolic pressure between adjacent sites (groin, thigh, just below knee, and at ankle) | >20 mm Hg decrease: possible occlusion >10 mm Hg: possible vessel calcification |
| Pulse volume recordings | Sharp peaks at each recorded site Similar across left and right extremities | Flattening on recording: occlusive disease |
| Transcutaneous oxygen pressures | >40 mm Hg suggest healing of ulcer or surgical incision is likely | <20 mm Hg predict nonhealing of ulcer or surgical incision |
| Duplex scanning | Low velocity ratio; constant hue and intensity of image | Velocity ratio >4 or peak velocity >400 cm/s indicates >75% stenosis |
High-resolution B-mode imaging combined with color and power Doppler and real-time spectral analysis have made duplex scanning one of the most widely used methods of noninvasive evaluation of arterial disease.59 Duplex scanning can accurately assess the location and degree of arterial disease from the level of the aorta to the level of the ankle. It provides accurate, objective information that supplements the physical examination. A disadvantage of this modality is the prolonged time it takes to perform an accurate examination of the entire extremity. Screening for arterial disease is done by comparing the signals from the various sites along the peripheral arterial tree and measuring the velocities at points of narrowing and comparing them with velocities in non-narrowed areas of the arteries.60 This velocity ratio correlates to a 50% diameter narrowing when the ratio value is greater than 2. When the velocity ratio is greater than 4, the corresponding arterial narrowing is greater than 75%. Peak velocities at sites of narrowing can also be used to estimate the degree of narrowing. The sensitivity and specificity of this test is on the order of 90% or greater.61 Although some surgeons may rely solely on duplex scanning for preoperative peripheral arterial bypass planning, many prefer to more directly outline arterial anatomy using other imaging options.
Two additional methods that successfully outline the specific arterial anatomy are MRA and CTA (Figure 19-3).62–65 Magnetic resonance imaging (MRI) detects radiofrequency signals given off by protons within a powerful magnet; such proton movement within body structures creates a detectable echo that can be imaged. Use of a contrast agent, such as gadolinium injected through a peripheral vein, greatly enhances the information gained from the images. MRA studies with gadolinium contrast are highly specific and 100% sensitive for identification of arterial lesions that require intervention.66 The visualization of the distal runoff arterial tree when there are multiple levels of occlusions is much better than that seen with conventional angiography; however, the spatial resolution of MRA images is less than that available through invasive digital subtraction arteriography (DSA). Calcium within the artery, which impacts operative planning, cannot be seen in MRA. Other disadvantages include the inability to use this magnet-driven modality in patients with pacemakers, defibrillators, and intracorporeal metallic fragments that might migrate.
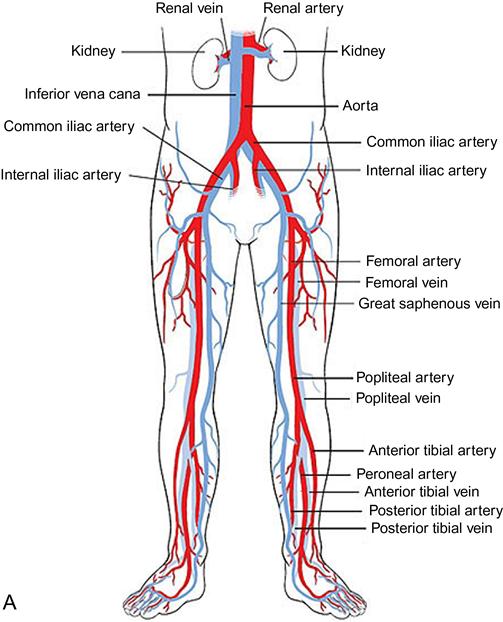
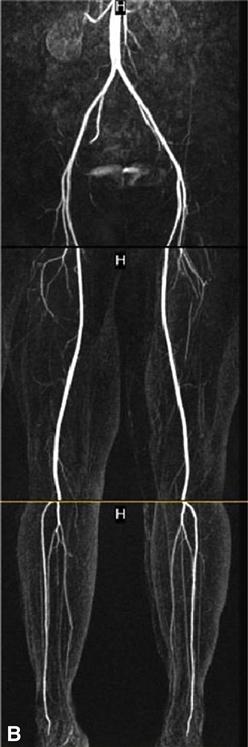
The advent of the helical or spiral CT scanners enabled CT technology to be applied to the diagnosis of peripheral arterial abnormalities due to the markedly reduced time required for scanning.67 With this technology, large areas of the body can be scanned in 20 or 30 seconds. An iodinated contrast agent is given through a peripheral vein. This avoids potential complications of a direct arterial injection but does not preclude the risk of inducing renal failure. Although less-concentrated contrast agents can be used for CTA, the volumes required are larger than those for conventional angiography. Both noncontrast images and postcontrast injection images are taken. One advantage of CTA over MRA is the excellent visualization of vessel wall calcium deposits with CTA. But if there is much calcium, these areas become indistinguishable from the areas opacified after contrast injection. CTA performed for evaluation of the arteries in the lower extremities is being increasingly used in clinical practice. However, its spatial resolution is less than that seen with DSA and small peripheral arteries are not as well visualized.
Vascular Examination: Invasive Strategies
Conventional arteriography and DSA are classified as an invasive examination strategies because both involve local surgical placement of a catheter into the femoral artery in the groin (or alternatively, the axillary, radial, or subclavian arteries, or the aorta), followed by introduction of radiopaque dye into the arterial tree and exposure to radiation.68
DSA computer-assisted x-ray method used to visualize vascular structures without superimposed bone and soft tissue usually seen on radiography.65 The computer subtracts initially recorded x-ray images from those taken following ion injection of contrast medium, to isolate arteries of the limb. Contrast material (dye) is injected into the arterial tree, often at the groin into the femoral artery to assess patency of distal vessels and evaluate collateralization. This carries some risk of cholesterol embolization from atherosclerotic plaques at the site of puncture if the artery is diseases at the injection site; in addition, many of the dyes used can be nephrotoxic.62 Despite these risks, DSA is considered the gold standard against which other less-invasive imaging methods are compared.
Because of the risks associated with invasive procedures, conventional arteriography is most typically used just prior to revascularization (bypass69 or thrombolysis70) or amputation surgery to determine specific location and severity of vessel occlusion as well as the extent of collateral circulation. It may also be used in place of MRA imaging for individuals with metal implants such as pacemakers and total joint prostheses. Because contrast material used in arteriography are nephrotoxic, this procedure is not appropriate for individuals with renal dysfunction; it is also contraindicated for persons with cholesterol emboli syndrome, or allergies to substances containing iodine.71,72
An invasive angiography procedure can take 90 minutes or more, depending on what is being imaged and how many images are required.73 During the procedure the area that is to be punctured is first numbed with a local anesthetic agent. If the person undergoing the procedure is particularly anxious, the physician can administer a sedative agent orally or parentally before or during the procedure. After the area is adequately anesthetized, an 18-gauge, 2.75-cm needle with a hollow, thin-walled outer cannula and a sharp, beveled, inner stylet is inserted into the artery. Once the artery has been punctured, the stylet is removed and replaced with a guidewire that is carefully threaded farther into the vessel; this wire then guides placement of the catheter that will deliver contrast medium. Catheters as small as 1-mm outer diameter may be used (3 French), but more commonly, 4 or 5 French catheters are used with outer diameters of 1.32 mm and 1.65 mm, respectively. Once 40 to 120 mL of contrast medium has been injected, the catheter is flushed with heparinized saline to reduce the risk of clot formation. Successive still or continuous video radiographs record the progression of the dye through the limb’s arterial tree (Figure 19-4). After the catheter is removed from the femoral artery, manual compression is held locally at the site for several minutes and the patients remain at bed rest for a minimum of 4 hours. To ensure healing of the puncture artery, physical activity (include rehabilitation interventions) is postponed until several hours to a day after the procedure.
Decision Making: Thrombolytic Therapy, Angioplasty, or Amputation?
Intraarterial infusion of thrombolytic agents is used to manage several situations: (a) acute thrombosis of the extremity, (b) following percutaneous angioplasty, (c) after surgical bypass procedure of an extremity, or (d) after acute thrombosis of a diseased native artery.74–76 The most commonly used thrombolytic agents are streptokinase, urokinase, and tissue plasminogen activator.77 Streptokinase and urokinase are non–fibrin-specific agents that convert circulating plasminogen to plasmin, which subsequently breaks down fibrin, one of the main components of clot. Tissue plasminogen activator is a fibrin-selective agent that acts locally by activating plasminogen to form plasmin, which, in turn, breaks up the fibrin in the clot. These agents are typically administered directly at the site of the clot (or as close as possible to it) via a catheter that is threaded through the arterial tree from the initial access site. Infusion may require several days; active rehabilitation begins after infusion is completed.
Intraarterial infusion of thrombolytic agents is typically followed by either systemic intravenous heparin therapy or oral warfarin therapy.78,79 For individual’s status after bypass graft, long-term warfarin therapy is used to reduce risk of graft occlusion. The heparins are a class of agents that bind to antithrombin III to effect anticoagulation. Warfarin blocks the action of the vitamin K-dependent factors in the coagulation cascade. This has implications for the patient undergoing active rehabilitation because these agents increase the risk of bleeding complications, especially with manipulation of the wound in the immediate postoperative period of about 7 to 10 days.
In the postoperative period, should an individual on anticoagulant therapy report a sudden and significant increase in pain at the bypass site or in the residual limb following amputation, the likelihood of local bleeding must be quickly evaluated.80 Outward signs of bleeding at the operative site, such as swelling, ecchymosis, hematoma, or active bleeding, may not be readily apparent should the bleeding occur at the subfascial level. Sudden onset of increased pain in the wound or extremity, along with recurrence of signs of limb ischemia, may also herald occlusion of the graft or progression of disease in the native vasculature following amputation at any level. It is important to identify signs of the failing graft as early as possible because the limb could possibly be salvaged if intraarterial thrombolytic therapy, as described earlier, is initiated soon enough.
Amputation is often the best option for some individuals with dysvascular or neuropathic disease when the arterial anatomy precludes bypass or if severity of disease is such that bypass cannot salvage irreversibly ischemic and gangrenous tissue, as well as persons with complex medical conditions at high risk for intraoperative and postoperative complications. Arterial bypass or thrombolytic therapy may be adjuncts to amputation so as to allow amputation at a more distal level, as long as there is no frank infection (wet gangrene) and the person’s arterial anatomy is conducive to bypass. In these cases amputation removes only nonviable portion of the limb, and the bypass or thrombolytic therapy improves the chance of healing at the more distal site.
Extensive gangrene of the foot, which is complicated by infection, is an indication for an open (guillotine) distal transtibial amputation. Time is given for the infection to be controlled by antibiotics and local wound care of the open surgical construct. The residual limb is then revised more proximally and a formal transtibial or transfemoral amputation with standard closure techniques is done.
Traumatic Injuries
Within the continental United States, traumatic amputations are the result of industrial and farming accidents, motor vehicle accidents, or injury during high-risk sport and leisure activities.81 In areas of the world where there is current or recent armed conflict, traumatic amputations are more likely to be the result of improvised explosive devices, land mines or grenades, shrapnel, or direct gunfire.82 Many of the injuries sustained by U.S. military personnel during the conflicts in Iraq and Afghanistan involve limb salvage or amputation.82 In most traumatic injuries the wound is likely to be contaminated by weapon fragments and organic materials (dirt, road debris, bits of clothing, vegetation), as well as damaged bone and soft tissue. In these situations the likelihood of infection as a consequence of introduced environmental microorganism, ischemia caused by vascular damage, and tissue necrosis because of direct damage is high.83 There may also be significant loss of blood volume and hypovolemic shock, head injury, chest or abdominal injury, and other fractures to contend with.
In emergency care the first priority is to stabilize the medical condition: maintain the airway, support respiration, and ensure adequacy of circulation to the heart and brain.84 The mangled limb is then carefully cleaned and debrided, a “cocktail” of systemic prophylactic antibiotics (e.g., cephalosporin, gentamicin, and penicillin) is begun, and a tetanus shot is given. Next the trauma team must decide whether the limb can be salvaged or replanted.85 The team considers many factors in making this difficult decision including severity of fracture and soft-tissue injury, likelihood of bony union, adequacy of arterial blood supply, neuromotor and sensory function of the extremity, potential for prosthetic use, recovery time, and anticipated long-term functional status and quality of life. Although there are a number of decision-making models available that attempt to quantify these dimensions when such a difficult decision is necessary (e.g., Predictive Salvage Index, Mangled Extremity Severity Score, Limb Salvage Index, Hanover Fracture Scale) most are more effective in determining need for amputation; few accurately predict the likelihood of a positive outcome of limb salvage.86,87
Limb Salvage
Limb salvage and replantation are associated with high health care costs because of increased likelihood of multiple hospitalizations for successive surgical procedures, complications and delayed healing, and secondary amputation if the salvage attempt failed.88 With improving microsurgical techniques, long-term outcome (e.g., return to work, perceived health status) is fairly similar when primary amputation and successful limb salvage and reconstruction are compared; however, residual impairment, functional limitation, and disability can have a significant impact on the quality of life in both groups.89,90 Any severe trauma to the lower extremities, whether salvage is attempted or amputation is the intervention of choice, is life altering.
In general, salvage attempts are most likely to demonstrate delayed healing or go on to secondary amputation if there has been high-energy injury, comminuted open fractures of Gustillo classification IIIC or higher, periosteal stripping, arterial injury with prolonged warm ischemia time, or complete disruption of the tibial nerve.91 Individuals or their family health proxy must be informed of the pros and cons of limb salvage versus amputation, including risk of infection and failure, as well as intensity and time frame for rehabilitation and likelihood of returning to premorbid functional levels.92
If the decision is made to attempt limb salvage, the individual is taken from the emergency department to the operating room.93 Arteriography (angiography) may be used to determine the extent of viable arterial perfusion. The next step is precise debridement of the damaged limb; surgeons must be sure that all compromised bone, muscle, and soft tissue are removed to reduce the likelihood of postoperative infection, necrosis, or later development of heterotopic ossification. It can be challenging to determine viability of cortical bone; cancellous bone is more likely to be revascularized. The biomechanical benefit of preserving as much bony length as possible must be weighed against the risk of developing osteomyelitis or delayed healing. Next, fractured fragments are stabilized with appropriate internal fixation, intramedullary rod, or external frame.
Defects in the bone may be filled with antibiotic-impregnated polymethylmethacrylate beads to further reduce the risk of infection and to preserve space for a later bone graft. The areas of the limb that were incised during surgery are stitched or stapled closed, while trauma-damaged areas may be temporarily closed with a biological dressing. The reconstructed limb is monitored closely, with sharp debridement of any nonviable tissue in the early postoperative period every 24 to 72 hours, as appropriate for the degree of wound contamination on initial injury, until a clean wound bed is obtained. Once the wound bed is consistently clean, the individual whose limb is being salvaged returns to the operating room (optimally within 1 week) for definitive closure of the surgical construct. This may be accomplished by delayed primary closure, split-thickness skin graft, local flaps, or, in some cases, vascularized pedicle graft. Subsequent to successful closure, the individual may return to the operating room for bone graft or transplantation or distraction osteogenesis to address osseous defects and limb-length discrepancy.
Amputation in Trauma Care
If limb injury is deemed too severe for salvage, a “guillotine” amputation may be performed to remove damaged tissues and restore hemostasis.83 If there has been significant contamination of the wound, the surgical construct may be left open (without skin closure) until the trauma team is satisfied that no infection has occurred.94 Once the individual’s medical condition has stabilized and the wound is clean, revision to a definitive level of amputation can be performed. Amputation after severe injuries may require split-thickness skin graft or pedicle graft to achieve adequate skin closure. Skin pressure tolerance when wearing a prosthesis can be problematic later in the rehabilitation process when any type of grafting has been necessary.
Traumatic amputation is most likely to occur in young and midlife adults who are otherwise healthy. Once damaged tissue is removed and wound closure is achieved, circulation is often adequate for successful healing. These individuals, however, may be more likely to develop depression in the postoperative period because the injury was so sudden, and amputation so unexpected.95 The sense of loss and alteration of body image after traumatic amputation can be more substantial than for an older adult with a diseased limb who “elected” to undergo amputation. Similarly, depression is a significant risk in those who are in the midst of limb salvage because of the likelihood of a long period of limited activity and altered appearance of the salvaged limb.
Neoplasm
Various tumors of soft tissue and bone range from slow-growing localized tumors to invasive and aggressive tumors (Box 19-1).96 Presenting signs and symptoms may include nonpainful enlargement of the limb, persistent pain, and pathological fracture.97 The incidence of tumors of bone and soft tissue demonstrate two age peaks: the first occurs in adolescents and young adults (especially osteosarcoma), with a second peak in mid- and late-life adults (especially metastatic).98
The most frequently diagnosed tumor of the skeletal system is metastatic, usually originating from a primary site in the breast, prostate, lung, or colon.99 The most common sites of metastatic bone disease are spine and long bones of the extremities. Metastatic cells disrupt and alter the balance of osteoblastic and osteoclastic activity within bone, resulting in higher rates of resorption and lysis of bony structure. Metastatic bone disease is most commonly manifested as a deep pain present at rest and during activity, and it carries an increasing risk of pathological fracture.100 Metastatic bone disease is typically managed with a combination of radiation and chemotherapy as well as nonsteroidal antiinflammatory or opioid medications to relieve pain and enhance function, and administration of bisphosphonate to reduce likelihood of recurrent skeletal-related events and recurreance.100–103 Some metastatic tumors may require an orthopedic surgical intervention such as placement of an endoprosthesis, along with orthotic intervention to preserve or restore function, especially if there has been pathological fracture with neuromuscular impairment.104
The incidence of primary malignant neoplasm involving bone and soft tissue is quite low when compared with tumors of other systems.97 Because of this, the management of primary tumors of bone has evolved into a specialty practice, which means that many newly diagnosed individuals are referred to regional centers where national guidelines ensure consistency of care.96 Although the number of amputations performed as a result of neoplasm is significantly less than those resulting from dysvascular/neuropathic disease, amputations as a consequence of neoplasm are more likely to be performed at proximal levels, including hip disarticulation. Along with trauma injury, neoplasm is the leading cause of limb loss in children and adolescents.
Decision Making: Limb-Saving Surgery or Amputation?
Until the 1990s most tumors of bone were managed by amputation with adjunctive chemotherapy or radiation.96 Currently, localized radical resection with allograph of bone, endoprosthesis, joint replacement or rotationplasty, along with and a combination of multiagent chemotherapy, isolated limb perfusion with tumor-necrosis factors, and radiation may be used.105–109 These strategies have significantly reduced the number of tumor-related amputations performed each year. Amputation may be necessary when there is large, multifocal, high-grade, proximally positioned sarcoma, especially if there has been pathological fracture or significant involvement of neurovascular structures and if the tumor is chemoresistant.110–114 Amputation is also performed when there is local recurrence following previous limb salvage.114
Limb-sparing intervention is often staged, requiring multiple surgeries, given the comorbidities associated with concurrent chemotherapy.115 In addition, the immunosuppression caused by chemotherapy increases risk of postoperative infection of the endoprosthesis; if such infections cannot be controlled medically, amputation of the limb is likely.113,116 Survival rates and rehabilitation outcomes are surprisingly similar for those with primary bone or soft-tissue sarcoma managed by amputation or by limb-sparing surgery and reconstruction, although quality of life may be compromised as compared with peers without sarcoma.117–119
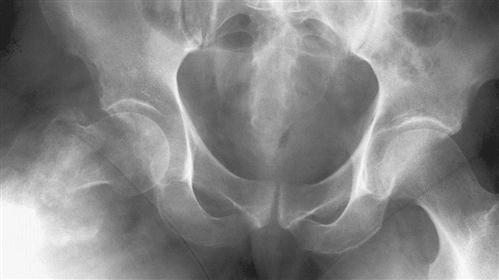
When a tumor of bone or soft tissue is suspected, initial assessment strategies may include a radiograph, bone scan, CT scan, or MRI.120 In individuals with previous history of cancer who present with bone pain, a radiograph may reveal a “moth-eaten” area within bone, with unclear boundaries or edges (Figure 19-5), demonstrating areas of osteolysis and reactive osteoblastic activity. A whole-body bone scan may be used to determine if there are multiple metastatic sites. A CT scan may be used to assess three-dimensional structural integrity and mineral content of the bone.
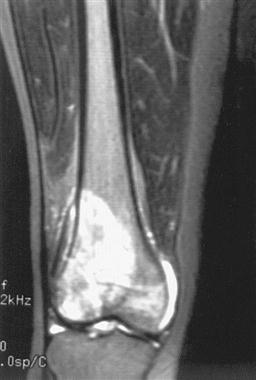
An MRI determines the extent of tissue involvement in marrow, bone, and surrounding soft tissue that is especially useful in planning surgical intervention for primary malignant tumor of bone or soft tissue (Figure 19-6).121 Laboratory tests may include blood assays, including sedimentation rate, C-reactive protein levels, serum electrophoresis, alkaline and acid phosphate levels, blood calcium levels, blood sugar levels, and hemoglobin concentration.122 For those with primary neoplastic bone disease, biopsy is used to determine cell type and contributes to pathological staging of the tumor. Depending on the tumor location, the surgeon may use an open frozen section, cannulated drill, or fine-needle aspiration to obtain a biopsy sample. For those with previous cancers of the breast, lung, bone, or colon, biopsy may not be necessary.
Once a primary tumor of bone has been typed and staged, the appropriate intervention is initiated.123 Nonpainful localized benign tumors typically do not require surgical intervention and are followed serially to watch for tumor progression. Symptomatic benign tumors (i.e., those that are painful or interfere with function) are treated by excision with bone graft or cement to fill the resulting bony defect.124 Localized malignant tumors are excised using limb-sparing and reconstructive strategies.108 Those with osteosarcoma or Ewing sarcoma almost always have preoperative and postoperative chemotherapy to minimize the likelihood of recurrence, while those with chondrosarcoma may not require such intervention.109,125
For successful limb-saving surgery, the surgeon must first completely remove the tumor, achieving wide, clean margins in the bone above and below the lesion. A tumor in the middle of long bone that does not include epiphyses is often excised en bloc and replaced with an allograft transplantation or expandable endoprosthesis.126 A tumor that crosses the epiphysis or extends into the joint space is also excised en bloc, and a total joint replacement performed to restore function of the limb.127 When the tumor is large, high grade, and invasive of other soft-tissue compartments of the limb, amputation may be the most appropriate strategy.110–114
Following limb-sparing surgery or amputation, appropriate rehabilitation interventions may include exercise to improve strength, range of motion, and endurance; energy conservation strategies; protective weight bearing during gait training with an appropriate assistive device; prosthetic rehabilitation (for those with amputation); functional training for mobility and activities of daily living; pain management (especially for those with metastatic disease); and patient and family education.128 The therapist’s understanding of the biomechanics and tissue manipulation undertaken in the reconstructive or amputation surgery is essential for appropriate exercise prescription and activity planning. Therapists must also be aware of the impact of chemotherapy and radiation treatments on healing soft tissue and bone; on peripheral sensation; and on physiological response to exercise and activity, as well as the individual’s overall health status, immune response, prognosis, and level of energy.129,130 The most successful rehabilitation programs are individualized and adapt to any adverse effects of concurrent adjuvant oncological interventions.131 Individuals with recent diagnosis of bone cancer often find significant support in interacting with others who have previously rehabilitated from limb-sparing or amputation surgery.132
Congenital Limb Deficiency
The birth of a child with a congenital limb deficiency is initially more distressing and disruptive to the new parents than to the infant.132 The development of the child’s body schema and personality is significantly influenced by the attitudes and expectations of parents and caregivers within the context of the social–cultural environment in which the child is raised.133 The rehabilitation, prosthetic, and eventual elective surgical management of children with limb deficiency is linked to age-appropriate developmental status, with the goal of enhancing function while minimizing deformity.134,135 The reader is referred to Chapter 29 for in-depth information on developmentally appropriate preprosthetic and prosthetic activities for mobility and skill, as well as discussion of the therapist’s and prosthetist’s role in counseling and educating the family and child about rehabilitation and prosthetic alternatives.
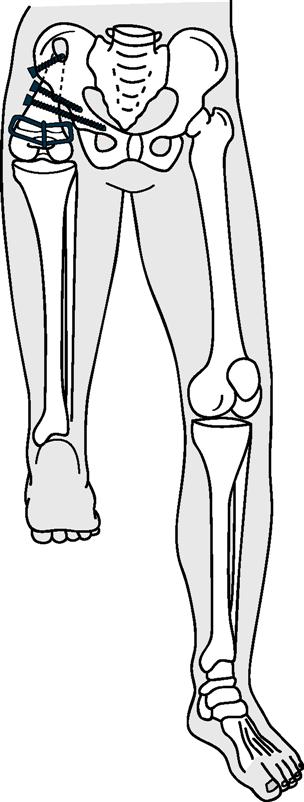
Orthopedic and surgical management of children with congenital limb deficiency focuses on enhancing appropriate growth of the residual limb, maintaining relatively equal limb length, enhancing joint function, and ensuring appropriate prosthetic fit. This may include custom-fit and fabricated orthoses or prostheses with shoe lifts; surgical and distraction procedures to lengthen bone; rotational osteotomy; simple surgical revisions to optimize shape of the residual limb; surgical correction of terminal overgrowth; and, when the deficiency is complex, conversion to a conventional level of amputation or disarticulation so as to enhance functional use of a prosthesis.136–140 School-age children with a partial longitudinal deficiency of the femur (proximal focal femoral deficiency) may be managed with a Van Ness procedure, a rotation osteotomy that fuses a tibia that has been repositioned 180 degrees to the residual femur (if present) or pelvis so that the foot can function as a knee joint (Figure 19-7), or with an osteotomy without rotation that is subsequently followed by ankle disarticulation to achieve a weight-tolerant residual limb closely resembling traditional transfemoral residual limb.141 Deficiencies of the fibula or tibia may be managed, depending on the severity of the defect and resulting deformity, by custom footwear and shoe lift, epiphysiodesis, ankle reconstruction or disarticulation, conversion to traditional transtibial amputation and prosthetic fitting, derotation osteotomy, and limb-lengthening procedures.142–144
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree