Demographic Imperative
The context for the increasing interest, and concern, about health care needs of older adults is found in demographic projections of an expanding elderly population in the United States and other developed countries. At the turn of the 20th century, one of every 25 Americans (4%) was 65 years of age or older. By 1994, this population had increased to one of every eight Americans (12.6%), or 33.2 million (
8,
9). While the elderly population grew 11-fold during this interval, the under-65 population only increased by a factor of three. Current projections indicate that 86.7 million, or one of every five Americans (20.7%), will be 65 years of age or older by the year 2050 (
10). By 2029, all “baby boomers” (Americans born post-WW II, between 1946 and 1964) will be 65 years or over (
10). Between now and 2030, the fastest growing segment of the population will be the 65 to 74 year olds, increasing from the current 6.3% to 10.4%. Between 2030 and 2050, however, the 75+ population will outpace the 65 to 74 cohort, reaching 11.6% of the population (every ninth American), while the 65 to 74 year olds will account for 9.0% (
10).
Distribution of the elderly is uneven across the United States, with 50% living in just nine states. While California has the greatest number of citizens over the age of 65, Florida has the highest proportion of elderly (18.6%) (
8). There are also ethnic aging trends, with projections that by 2050 the proportion of older white individuals will have decreased to 67% (from 87% in 1990), with corresponding increases of older Hispanic and black American citizens of 16% and 10%, respectively.
This demographic phenomenon is not limited to the United States, as a number of developed countries (including Italy, Japan, Germany, Sweden, and Great Britain) show 20% or more of their populations over age 65 (
8). As of 1994, there were 357 million older people worldwide, representing 6% of the total population.
There is increasing recognition of differences in health care needs and issues among subgroups of older people. Of particular significance from a health care standpoint is the rapidly expanding relative proportions of the population age 65 years and older who are 75 to 85 years of age (old old) and 85 years of age or older (oldest old). These groups include many of the so-called “frail elderly,” with a disproportionately high prevalence of disabilities and consumption of health services (
11). Another related dynamic is recognition of increasing racial and ethnic health disparities among older Americans, as the relative proportions of racial and ethnic minorities increase: from 2003 to 2050 the proportion of Non-Hispanic whites will decrease from 83% to 61%, while Hispanics will increase from 6% to 18%, blacks from 8% to 12%, and Asians from 3% to 8% (
12).
Compression of Morbidity
Older people also are living longer: 1990 statistics estimated a longevity at 65 years of age of 15.0 years for men and 19.5 years for women; this is projected to increase further to 17.1 and 22.6 years, respectively, by the year 2040 (
13). Contributing factors to these increases in longevity include improved access to health care, advances in medical care, overall healthier life-styles, as well as better health prior to age 65 (
14). The increasingly delayed occurrence of death at all ages appears in part to be due to reduced lethality of such diseases as stroke, cancer, and myocardial infarction, resulting from risk factor reduction as well as improved health care interventions (
9). Increasingly, people are surviving their initial encounter with these previously fatal diseases, resulting instead in chronic illness. This trend has been termed the fourth stage of epidemiologic transition (i.e., the postponement of death from degenerative diseases) (
15). These significant reductions in mortality are associated with an increasing risk for development of various chronic diseases. Certainly, the incidence and prevalence of many potentially disabling chronic illnesses increase substantially among older adults, including arthritis, osteoporosis with associated fractures, stroke, amputation, and various neurodegenerative disorders (e.g., Alzheimer’s disease, Parkinsons disease) (
2,
10,
11,
13).
This demographic imperative has far-reaching implications for increasingly limited U.S. health care resources and dollars: current national direct cost of medical services for older individuals with chronic conditions is in excess of $470 billion (in 1990 dollars), with a projected near doubling by the year 2050 (
13). In 2004, the average annual health care expenses were $8,900 for the 65+ population, compared to $3,028 for the under 65 population (
10). The old-old and oldest-old groups consume the greatest proportion of resources, and a disproportionate amount of these health care costs represent nursing home and other institutional care (
11). While the overall proportion of elderly individuals residing in nursing homes decreased from 6.8% in 1982 to 4.2% in 1999, these rates vary dramatically by age (
16). Only 1% of young old (65 to 74 year olds) reside in nursing homes, contrasted with 20% of the oldest old (85 years of age or older) (
9). In fact, the latter group comprises 45% of all elderly nursing home residents.
From another perspective, however, the vast majority of the 85-or-older population is not in nursing homes, and half of those in nursing homes do not necessarily need to be there because they have potentially preventable (or reversible) disabilities related to their chronic disorders (
17). In fact, it appears that much of the increased health care cost associated with aging (across all health care settings) is significantly related to activity limitation, rather than chronic disease (
18). Fortunately, there is increasing evidence that disability among older individuals may be decreasing (
16,
19). Reports of limitation of activity due to a chronic condition in the 65+ population have continued to decrease, from 38.7% in 1997 to 32.6% in 2006 (
12).
These findings continue to lend credence to the concept advanced by Fries in 1980 of “compression of morbidity,” in which he predicted that if the age of onset of disability could be significantly delayed (e.g., with regular exercise, healthier diets, elimination of smoking, improved health care interventions) in the context of a relatively “fixed” life span, then terminal predeath disability could be compressed into a shorter interval (
20). He postulated that health care needs for older people would decrease because they would be relatively healthy and functional until shortly before their death. A related prediction was that this anticipated short duration of predeath morbidity, and accompanying disability, would be expected and accepted with acknowledged futility of medical intervention. There is consistent evidence of increased health care costs associated with aging, with one study documenting nearly one half of life-time health care costs occurring after age 65 (
21). Interestingly, although a number of reports have documented dramatically increasing health care costs near the end of life, it should be noted that these are costs of dying, not of aging
per se (
22). Further, there is evidence suggesting that the incremental costs associated with extending life may actually plateau or even decrease (
23,
24,
25). These findings are of critical significance in the context of increasing focus on cost containment and debate over the feasibility and appropriateness of rationing of health care (
26). Twenty years after his initial predictions, Fries (and others) cites increasing evidence in support of the trend of compression of morbidity, even though the mechanisms are not clear (
27,
28). He reiterates the importance of a research agenda focused on delineation of the epidemiology of disability, determination of the fundamental basis of age-associated chronic conditions, and identification of effective interventions for preventing or delaying resulting disability (
27). This call to action has been echoed by others as well (
1,
2,
21). On the other hand, Kane raises disturbing questions regarding potentially adverse economic, cultural, and individual consequences of successfully overcoming the aging (and dying) process and urges ongoing dialogue to further explore these ethical questions (
29).
Active Life Expectancy
A derivative of research into longevity and epidemiology of aging relates to issues of quality of life, given the increased incidence of frequently disabling chronic disorders such as degenerative neurologic diseases (e.g., Alzheimer’s disease, Parkinson’s disease), degenerative musculoskeletal conditions (e.g., osteoporosis, osteoarthritis), and multisensory losses (e.g., cataracts, presbycusis). One concept that attempts to delineate quality of life for older individuals has been termed “active life expectancy,” referring to the proportion of remaining life span characterized by functional independence (
30). This concept, also referred to as “disability-free life expectancy,” has been expanded to consider both physical and cognitive impairments, as well as their interrelationships (
28,
31). A significant gender difference in active life expectancy with aging has been identified. As can be seen in
Table 59-1, older men have a greater proportionate active life expectancy at all ages. However, due to greater longevity, older women enjoy longer actual durations of active life expectancy than older men, until age 85 (
31,
32). A recent longitudinal British study confirmed a gender difference, with men aged 65 showing 79% active life expectancy (12.1 of 15.3 years) and women aged 65 showing 57% active life expectancy (11.0 of 19.4 years) (
28). Further investigation into
dynamics impacting active life expectancy reveals the deleterious effects of diabetes (decreased total life expectancy and active life expectancy at all ages, with a 25% reduction in active life expectancy in 85 year olds) and depression (reduction of active life expectancy by 6.5 years in 70-year-old males and by 4.2 years in 70-year-old women) (
33,
34).
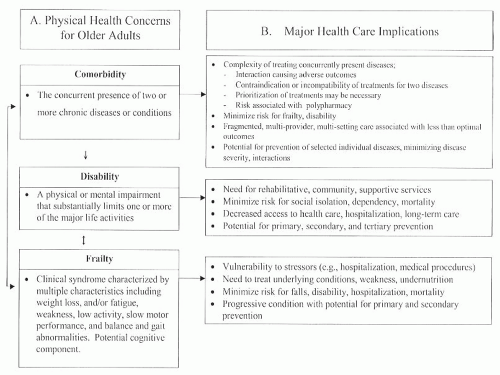
Comorbidity, Frailty, and Disability
Although the increasing incidence and prevalence of (often multiple) chronic diseases with aging is well documented, there is no one-to-one correlation between either disease and illness (
35) or disease and disability (
19). A significant proportion of older people are limited in the amount or kind of their usual activity or mobility secondary to chronic impairments: over 60% of adults with functional impairments due to chronic health problems are 65 years of age or older (
13). However, disability in this older population can also be very dynamic, with frequent transitions between periods of independence and disability and levels of disability (
36,
37). Also, the overall health of progressive cohorts of older persons has been changing. Although there have been predictions that future generations may well be healthier than current generations, due in part to higher levels of education and health awareness (
36), there are disturbing trends of increasing obesity and diabetes in older adults, with significant associated morbidity and mortality (
10).
Fried et al. have helped to clarify the dynamics of interrelationships among comorbidity, frailty, and disability—terms frequently used interchangeably—by providing discrete definitions of each entity and describing the synergistic impact of each on the other(s) (
38). As seen in
Figure 59-1,
Disability accordingly is defined as “difficulty or dependency in carrying out activities essential to independent living” (such as activities of daily living [ADLs], mobility, instrumental activities of daily living [IADLs]), while
Frailty is “a physiologic state of increased vulnerability to stressors that results from decreased physiologic reserves, and even dysregulation, of multiple physiologic systems.” Frailty appears to represent an aggregate expression of risk resulting from age- or disease-associated physiologic accumulation of subthreshold decrements affecting multiple physiologic systems (
38). Fried cites evidence in support of a phenotype of the clinically frail older adult, characterized by the presence of a critical mass of three or more “core elements” of frailty (weakness, poor endurance, weight loss, low physical activity, and slow gait speed) (
39).
Comorbidity is commonly defined as the concurrent presence of two or more disease processes in the same individual. Fried suggests that comorbidity is the aggregation of clinically manifested diseases present in an individual, while frailty is the aggregate of subclinical losses of reserve across multiple physiologic systems (
38).
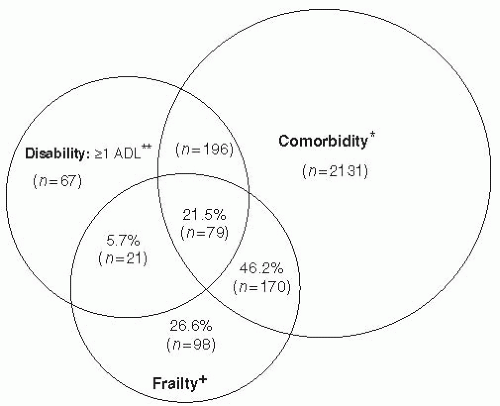
Fried further documents the interrelationships between frailty, comorbidity, and disability (
Fig. 59-2), noting that frailty and comorbidity each predict disability, while disability appears to exacerbate frailty and comorbidity (
38). While medical care for each of these entities is typically complex, particularly when they coexist, Fried specifically notes that rehabilitation interventions for disabled older adults to regain function and/or prevent further functional decline need to factor in recognition and treatment of frailty and comorbidity to maximize likelihood of success. She cites growing evidence to suggest that frailty, comorbidity, and disability may be preventable, but with different intervention strategies (
38). The importance of prevention is reinforced by the clear association of each entity, especially when concurrent, with higher health care costs and poorer survival (
38,
40). Recent research is further differentiating between various subtypes of disability in older individuals (transient, short-term, long-term, recurrent, and unstable) and their relative clinical significance (
41).
In summary, an increasing proportion of older people are living longer and are at increased risk of developing varying (and changing) degrees of comorbidity, frailty, and functional losses with disability. The challenge for health care providers, accordingly, is to try to prevent the onset/progression of these entities with early and effective medical and rehabilitation interventions, to reverse or at least minimize their deleterious effects on health and function.
Successful Aging
Distinctions have been made between aging processes representing “primary aging” (i.e., apparently universal changes that occur with aging, independent of disease and environmental effects) and “secondary aging,” which includes lifestyle and environmental consequences and disease associated with the aging (
42,
43). A number of tenets associated with aging research are being reexamined, particularly with the observation that a pathologic process may exaggerate an aging process believed to be normal, even before the disease is detected clinically (
42). There is increasing evidence that the nonpathologic processes of aging are distinct from, but not necessarily independent of, the pathologic processes of disease (
38,
44).
Most studies of normal aging have focused on the physiological and biochemical changes occurring with aging, with explicit exclusion of disease. However, it is increasingly apparent that such factors as personal habits (e.g., diet, exercise, nutrition), environmental exposures, and body composition may have significant impact on observed aging changes (
43). Rowe has proposed a conceptual distinction between “successful aging” and “usual aging” (
45). He suggests that “successful aging” could be characterized by minimal or no physiologic losses in a particular organ system and would comprise a relatively small subset of the total “normal” (i.e., nonpathologic) aging population. The remaining majority of “normal” older adults demonstrate “usual aging,” with gradually progressive but significant declines in various physiologic functions.
The significance of this concept lies in the implications for modifiability of usual aging by virtue of addressing such variables as level of physical activity, diet and nutrition, and environmental exposures (
27,
43,
44). This principle is demonstrated in studies documenting the effects of exercise, diet, and drugs on the usual aging observations of carbohydrate intolerance. Rowe proposes that geriatric research into health promotion initiatives concentrates on increasing the proportion of older adults who “successfully age” by identifying and modifying extrinsic risk factors contributing to “usual aging” and decreasing the manifestations of “pathologic aging” by preventing or minimizing adverse effects of acquired disease processes (
45). This would reinforce the previously described concept of compression of morbidity, with greater active life expectancy. Indeed, studies are helping to determine which factors distinguish high-functioning older adults from other populations of older adults (
28,
33,
43,
46).
Theories of Aging
With continuing research, it appears likely that there is no single cause of aging (
44,
47). The current concepts of aging characterize the process as extremely complex and multifactorial and suggest that various theories of aging should be viewed not as mutually exclusive, but rather complementary (
47). From this perspective, hypotheses based on passive (i.e., random) and/or active processes of genetic programming could be considered in conjunction with superimposed nongenetic
mechanisms (e.g., environment, lifestyle), producing varying individual vulnerability (
42,
44,
45,
48). Certainly this would help explain the well-documented phenomenon of differential aging, whereby individuals of the same species appear to age at different rates (
44,
46). Multiple levels of research suggest that rates of aging are affected to varying extents by heredity, lifestyle, environment, occurrence of disease, and psychological coping abilities (
17,
32,
42,
46).
Active investigation continues in the areas of neuroendocrine pacemakers, telomere shortening, and attenuation of inducible stress responses (
48,
49,
50,
51). A number of studies have also focused on the phenomena of apoptosis and autophagy, related to cell death of proliferating and postmitotic cells respectively, as well as mitochondrial degradation in long-living cells (
52,
53). There is some evidence suggesting that pathologic stimulation of apoptosis may result in a number of degenerative disorders commonly associated with aging, whereas inhibition appears to be associated with a variety of forms of cancer (
52).