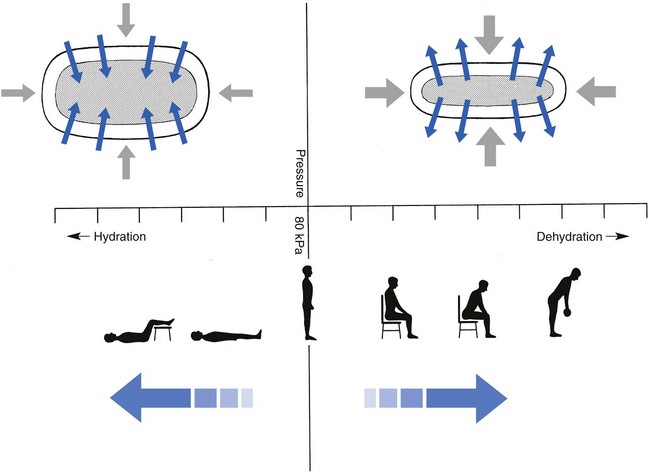
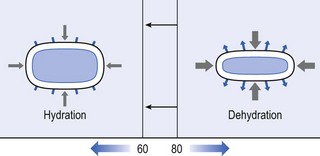
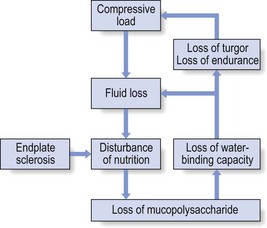
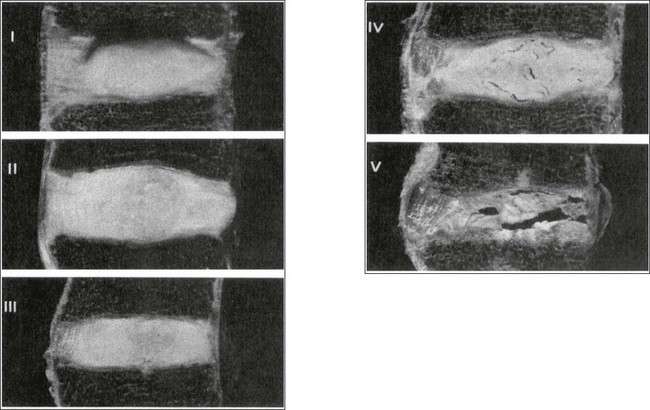
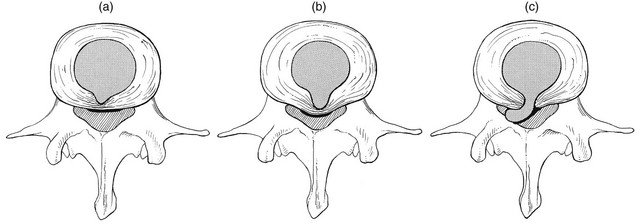
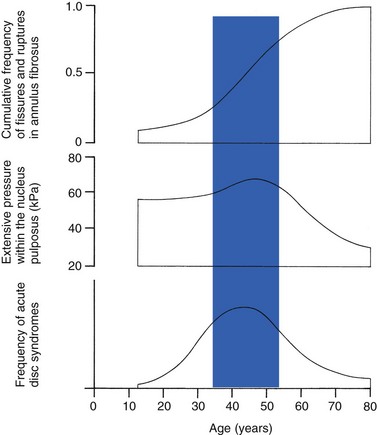
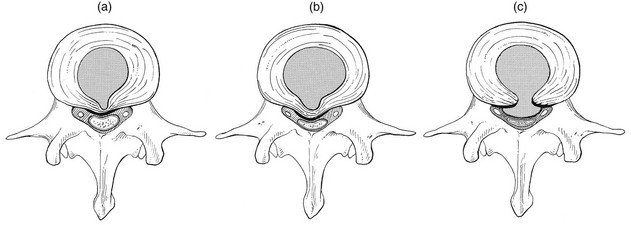
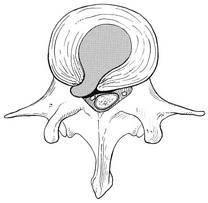
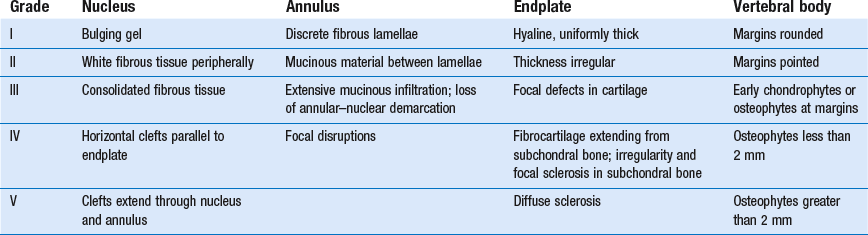
32 The human spine functions in a reverse way to that for which it was originally developed: instead of a hanging bridge connecting the anterior to the posterior limbs of a quadruped, it has become a vertical column of a biped, fundamentally changing the biomechanics and imposing larger loads on the lower lumbar discs. Humans’ upright posture has long been blamed as the main cause of the development of early disc degeneration: ‘intervertebral disc disease is the price we pay for the gift of the erect posture.’1 Disc degeneration in animals is very unusual.2 Neither have localized bulging and Schmorl’s nodules ever been observed in quadrupeds.3 However, a quadruped forced to adopt an erect position quickly develops structural changes in the annulus fibrosus and nucleus pulposus.4–6 Large disc herniations at the lumbosacral level also occur in 25% of bipedal rats.7 It seems obvious, therefore, that the upright position and the consequent increase in static loading of the disc8 are the main causes of the early and progressive development of degeneration in the human lumbar spine. Degeneration of the lumbar discs starts very early in life. Clear microscopic signs of degeneration have been demonstrated in a 4-year-old.9 Several reports of prolapsed discs in children and adolescents show that major disc degeneration appears at or before puberty10–14 and it has been maintained that after the age of 30 years there is no lumbar disc which does not show some degenerative changes.15 There seems to be no way to escape this degeneration because it affects all, whatever their weight, physical build or athleticism.16 Both the erect posture, which imposes heavier mechanical stresses on the spine, and the decreased regenerative capacity of the disc make it very vulnerable. As has been described (see Ch. 31), the disc depends for its nutrition on diffusion through the endplates and annulus, which can only take place as an outcome of change in intradiscal pressure within a physical range (Fig. 32.1). Consequently, both excessive forces and immobilization will have deleterious effects on disc tissue.17 A modern, mostly sedentary lifestyle, for example, puts continuous increased pressure on the disc; it is then constantly kept low in water content, which prevents the transport of nutrients. As the cells are undernourished, the fibrils and ground substance become damaged and their chemical composition changes.18 Enzymatic depolymerization of collagen and mucopolysaccharide causes a decrease in weight and content that results in a drop in oncotic pressure, which leads to further loss of water.19 The drying out of the disc is the most characteristic sign of intervertebral degeneration. The loss of hydrostatic pressure in an ageing disc is also reflected in the change in daily variations in body height: in adolescence the difference in body height between morning and night is 2%, but this drops to 0.2% in adults.20 The mature disc is thus permanently dried out and lacks the osmotic elasticity of the young disc. Loss of water-binding capacity is also responsible for the decrease of disc turgor and loss of endurance. The disc does not sustain the normal loads and the hydration–dehydration curve shifts in the direction of further dehydration (Fig. 32.2). A vicious circle of further degeneration, further decrease in molecular weight and further fall in water-binding capacity is set up (Fig. 32.3).21 With advancing age, the permeability of the vertebral endplates also decreases. The accompanying calcification compromises the nutrient supply to the adjacent nucleus, so enhancing further disc degeneration.22,23 The early stage of the changes in vertebral bone structure is first seen on magnetic resonance imaging (MRI).24 Vertebral endplate sclerosis later becomes radiographically visible.25 The intervertebral disc is characterized by a tension-resisting annulus fibrosus and a compression-resisting nucleus pulposus. As the nucleus pulposus dries out, it becomes less able to exert fluid pressure. The annular fibres, no longer under constant tension in relation to the nucleus, bear a greater share of vertical load and are subjected to bulk deformations and shear strains.26 This leads to tears in the annular fibres.27 The posterior annulus, especially the area between annulus and nucleus, is subjected to most of the mechanical strain.28 As this posterior boundary zone is also most exposed to nutrient deficiency,29 it will be the first to wear through, so that small radial fissures appear.30,31 Further softening and loosening of annular tissue leads to expansion of these fissures and the formation of cavities and cysts, which are radiographically seen as air pockets.32 Nuclear displacements through these fissures will lead to a further decrease in disc height. As a result, the intervertebral joint becomes unstable and is subjected to more bulk deformation and more shear strain. Ruptures permit further migration of the disc material in the direction of least resistance (mostly backwards). The result is intradiscal displacements, bulging beyond the vertebral borders and disc prolapses (see Ch. 33). Ageing of the disc also involves structural changes in the endplate. Between the ages of 20 and 65, the endplate becomes thinner and cell death occurs in the superficial layer of the cartilage.33 The vascular channels in the subchondral bone of the endplate are gradually occluded, finally leading to complete endplate sclerosis. As the endplate plays an important role in the nutrition of the disc, changes in it will contribute to further degeneration of the whole intervertebral disc. Also, the strength of the vertebral endplate decreases with age, which renders it more liable to (micro)fractures. Microscopic defects may be followed by invasion of the disc by young blood vessels and connective tissue cells.34 The latter undergo metaplasia into cartilage cells and granulation tissue, and adult connective tissue also forms.35 This ‘invading’ connective tissue can spread out into the disc, create zones of decreased resistance and cause further weakness and instability. It has even been hypothesized that this ‘new’ proliferative tissue plays a part in disc herniations.36 The macroscopic degeneration of the disc was divided into four grades by Friberg and Hirsch37 and later by Nachemson.38 This scheme for assessing the gross morphological changes in the lumbar intervertebral discs has recently been improved by Thompson et al,39 who consider five morphological grades (Table 32.1 and Fig. 32.4). Table 32.1 Description of the morphological grades Reproduced with pernission from Thompson et al.39 Macroscopic grades of disc degeneration have been correlated with age, sex and spinal level for 600 lumbar intervertebral discs from 273 cadavers in a review by Miller et al.40 Their conclusion was that, by the age of 50 years, 97% of all lumbar discs exhibit degeneration and that the L3–L4 and L4–L5 levels usually contain the most severely degenerated discs. Another study demonstrated that there is disc degeneration in at least one lumbar level in 35% of subjects between 20 and 39 years of age, and that all subjects 60–80 years of age show disc degeneration.41 These figures establish that disc degeneration is a normal age-dependent pathophysiological process; it therefore cannot be regarded as a ‘disease’ but is a normal biological event from which no one will escape.42 Fissures in the annulus fibrosus will inevitably lead to changes in the biomechanical behaviour of nucleus and annulus, which can then result in intradiscal nuclear displacements, protrusions and prolapses (Fig. 32.5). The nucleus and inner annulus do not contain free nerve endings. Displacements of these structures are therefore painless, except when they irritate other more sensitive tissues, such as the outer annulus, the dura and the nerve root sleeves. Therefore it is not the size of the migration that is important but the degree of irritation of the pain-sensitive tissues. Because imaging techniques (computed tomography (CT), CT discogram and MRI) can only detect the size and localization of the bulges, and not always their effect on surrounding tissues, many more disc displacements are detected during imaging than actually cause symptoms.43,44 More than 50% of asymptomatic patients over 40 years of age have either alterations in or displacements of disc.44 Single or multilevel disc bulging is visible on the MRIs of between 28 and 85% of the adult population who do not have activity-limiting low back pain.45–48 Cyriax49 listed five different directions in which discal tissue can move: posterior, posterolateral, anterior, vertical and circular. Each type of displacement occurs during a specific period in the degeneration of the disc. Posterior and posterolateral displacements are typical in the third, fourth and fifth decades. Anterior displacement resulting in osteochondrosis is a disorder of teenagers, whereas anterior displacement in combination with disc resorption is a lesion of the elderly and is seldom seen below the age of 50. It is surprising to find that low back syndromes (lumbago, backache, sciatica) are age-related,50,51 whereas the incidence of fissures, ruptures and degeneration of the disc increases linearly during the whole of life. Extensive pressure within the nucleus pulposus is higher in individuals between 30 and 50 years of age than in the elderly (Fig. 32.6).52 The conclusion is that posterior displacement of the disc will be more likely to appear when increased intradiscal pressure coincides with some damage to the posterior annular fibres. In other words, the combination of intradiscal pressure and lowered resistance of the annulus fibrosus is the main biomechanical factor contributing to protrusion–prolapse. When the expansive forces of the nucleus pulposus decrease and, because of the loss of water, the disc gradually deflates, the tendency to displacement diminishes.53,54 Other factors protecting the ageing disc against displacements are increasing stiffness of the posterior ligaments, which limit spinal mobility, and the formation of osteophytes, which distribute the load over a larger area. The posterior boundary zone between the annulus and the nucleus is the first part to develop areas of degenerative change and fissures (see p. 438). In sitting and forward bending, the disc is asymmetrically loaded, with a compression strain anteriorly and a tensile stress dorsally. Because of the increase of intradiscal pressure, the nucleus pulposus is pushed dorsally and applies more stress to the weakened posterior fibres, which are already subjected to the strong tensile stress imposed by the flexion.55 Posterior migration of disc tissue beyond the posterior limits of the intervertebral joint space causes tension on the outer annulus and the posterior longitudinal ligament. Irritation of ligament, dura mater and the Hofmann complex combines to produce backache (Fig. 32.7a). The patient’s history usually suggests the type and localization of the displacement and the degree of dural irritation: progressively increasing backache usually stems from a nuclear displacement. A sudden shift indicates that the material that has moved is of harder, annular consistency. If the bulge irritates the dura centrally, the pain is central, probably with some bilateral gluteal radiation. If it lies more to one side, unilateral pain and pain in one buttock result. Further irritation of the dura mater results in a clinical picture that is called acute lumbago (Fig. 32.7b). In a cartilaginous displacement, the patient states that bending, lifting or standing, after sitting for a while, resulted in acute and severe pain. Alternatively, the pain and disability have come on gradually, some hours after a period of stooping or sitting, which suggests protrusion of part of the nucleus. In both events, a constant ache is experienced which forces a pain-avoiding position, usually in flexion and/or lateral deviation. The pain is agonizing and radiates to one or both legs in an extrasegmental way. Dural symptoms (pain on coughing and sneezing) are positive, as are dural signs (limited straight leg raising and lumbar pain on full neck flexion), indicating that the dura is under constant and severe stress. A massive posterior protrusion may cause a rupture of the posterior longitudinal ligament. The entire cauda equina will then be compressed between the protrusion and the laminae. This not only squeezes the nerve roots on both sides but also causes compression and atrophy of the centrally localized S3 and S4 roots, with disturbance of bladder and rectal function (the S4 syndrome or cauda equina syndrome) (Fig. 32.7c).56,57 Root pain results from pressure of the prolapsed or protruded disc against the dural sleeve of the nerve root and subsequent irritation of the latter (Fig. 32.8). This may be primary or secondary. Because of its high oncotic and hydrostatic pressure, the young disc sometimes invades the vertebral endplates. This occurs particularly in the regions where the endplates have low resistance against increased anterior loading.58,59 The nucleus pulposus may then move forwards between the cartilaginous endplate and the bone of the vertebral body, causing injury to the growth zone of the vertebra (Fig. 32.9). The result is a developmental deformity of the anterior part of the vertebra. During the course of this, protrusion may separate a small triangle of bone at the anterior corner of the vertebral body (limbus vertebra).60 If the osteochondrosis involves several consecutive vertebrae, a kyphotic deformity results (Scheuermann’s disease).61 Such deformities occur only during the period of spinal growth and are asymptomatic. Care must therefore be taken not to overemphasize the significance of incidental radiological findings.62 Tears at the anterior and anterolateral part of the disc lead to both anterior and anterolateral herniation. Anterior displacement of the disc causes dissection of the anterior longitudinal ligament from its bony attachments, which stimulates bony outgrowths along the anterior and anterolateral aspects of the vertebral body. These first appear in a horizontal direction and then vertically, following the course of the anterior longitudinal ligament (see p. 423). In advanced cases, the whole disc is displaced anteriorly. On a lateral radiograph this can be seen as two ‘beaks’ containing the disc, while the intervertebral space is so narrowed that the vertebral bodies come to lie in apposition.63
Ageing of the lumbar spine
Introduction
Ageing of the disc
Mechanism
Structural changes
Disc displacements
Posterior displacement
Posterolateral displacement
Anterior displacement in adolescents
Anterior displacement in the elderly
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Ageing of the lumbar spine