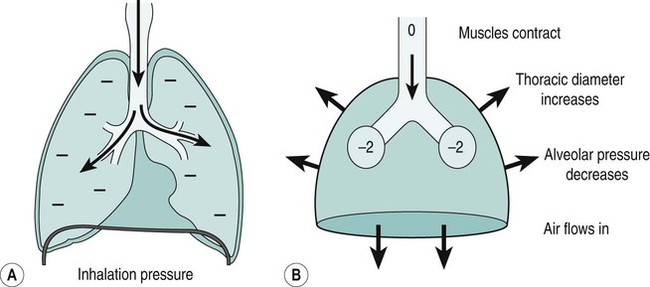
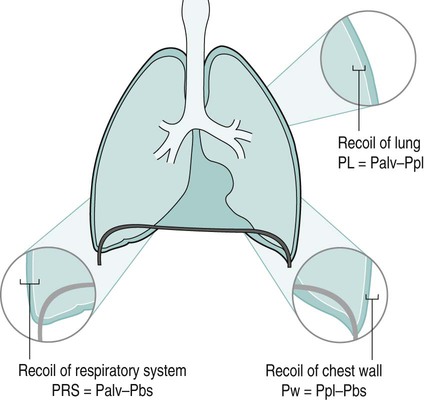
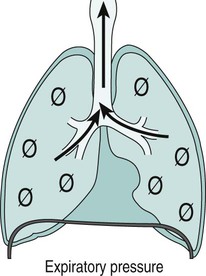
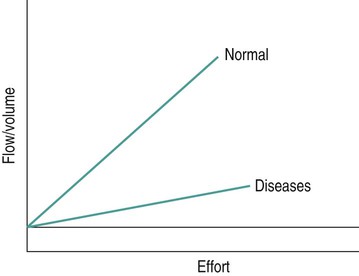
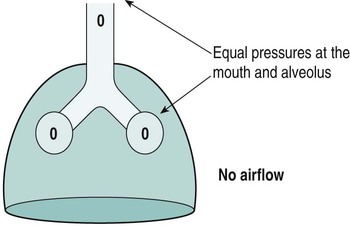
Sue Pieri Davies, Helen Carruthers This section, focussed on conventional mechanical ventilation, should be read in conjunction with Chapters 6 and 8. It is beyond the scope of a single chapter to detail all appropriate systems, the numerous conditions and causes of ventilatory failure, the concept and uses of non-invasive ventilation, and the more specialised area of domiciliary ventilation. A more detailed knowledge of these subjects can be obtained through further private study of the recommended texts and websites provided at the end of this chapter. The respiratory muscles consist of two main groups: the primaries (consisting mostly of the diaphragm and intercostals) and the accessories (comprising mostly of the scaleni, abdominals and sternocleidomastoids). It is also important to remember the effects upon the oxygenation status and the balance of the blood pH with increasing metabolic demands should the recruitment of other additional muscles, for example the facial muscles with pursed-lip breathing, and the shoulder girdle and arm fixators, be required during times of respiratory distress/dysfunction. The primaries’ main role is that of ventilation. The accessories have other functions, but are recruited to facilitate ventilation when required. Normally, the respiratory muscles have both ventilatory and non-ventilatory motor functions, for example the diaphragm acts as the primary respiratory muscle, responsible for generating approximately 60–70% of the tidal volume while also being responsible for raising intra-abdominal pressure for postural stabilisation of the torso, parturition and micturition. Such considerations must be appreciated by the therapist: when the respiratory muscles are required for both motor and ventilatory functions, their ability to assist ventilation is reduced. This is of particular importance when ventilatory support has recently been reduced and motor activity is being encouraged during daytime hours, i.e. during the weaning and rehabilitative phases of recovery. Upper limb strengthening exercises may be a primary aim at this stage so increasing ventilatory support overnight may be appropriate (Table 7.1). Table 7.1 Comparison of skeletal muscle fibre types (Reproduced from Schauf et al. (1990), with permission.) The respiratory muscles share the common features of other skeletal muscles and consist of a mixture of fibre types (Johnson et al. 1973). The proportions of fibre types and the metabolic constituents (e.g. capillaries; glycolytic and oxidative capacities; and time of recruitment in contraction) determine a muscle’s strength and endurance properties (Schauf et al. 1990). Type I fibres are important for endurance (slow twitch, high oxidative capacity are recruited first and are most resistant to fatigue). Type IIa fibres have a higher oxidative capacity, fast twitch and produce an intermediate level of force, and so are relatively resistant to fatigue, while Type IIb fibres have a low oxidative capacity, fast twitch, produce the greatest force on activation, are the last to be recruited for motor efforts and are easily fatigued when used repeatedly. Greater knowledge of muscle physiology is required if the aim is to train the respiratory muscles, rather than rest them (via ventilatory support). Muscle training may be an appropriate physiotherapy intervention to facilitate the weaning episode of the prolonged ventilatory supported individual, where muscle wasting and disuse atrophy are evident, though more research is required in this area. Normal resting ventilation is a tri-cyclical activity consisting of the inward flow of air (inspiration), the outward flow (exhalation) and the rest phase, which constitutes the zero-flow status. During the inspiratory and expiratory cycles, a volume of air moves in and out of the lungs. These changes occur as a result of pressure gradients between the airway opening (or mouth) and the alveoli. Prior to the beginning of inspiration, the pressures at the airway opening and in the alveoli are equivalent. As there is no pressure gradient, there is no air movement – this is known as the resting period of the respiratory cycle, where air neither enters nor leaves the lungs (see Figure 7.1). As inspiration begins the respiratory muscles contract, causing an upward and outward movement of the chest wall (the bucket and pump handle effects) and the diaphragm descends (see Figure 7.2). This is known as the active phase of the breathing cycle and demands effort (termed the work of breathing). The changes in thoracic dimensions create a drop in the alveolar pressure; the pressure gradient between the airway opening and the alveoli results in an inward movement of air. The volume change that occurs is called the tidal volume and is, on average, 500 mL. (Joint guidelines by the British Thoracic Society and Association of Respiratory Technicians and Physiologists are available for a detailed underpinning of spirometric values and tests. Also downloadable from the internet are the American and European thoracic society recommendations for spirometry). The work of breathing derives from the two resistive forces of the lungs and chest wall, i.e. the elastic (see Figure 7.3) and frictional forces. Forces within the respiratory system that oppose inflation of the lung and therefore ventilation can be grouped into two categories: The pressure change that is generated on inspiration must be sufficient to overcome such forces. The effort required and the resulting volume change is termed the ‘work of breathing’. Normally, the work of breathing is minimal (healthy lungs). A pressure gradient of 2–5 cmH2O is typically needed to move the average tidal volume. Expiration is mostly passive as a result of the elastic forces returning the lungs and chest wall to a balanced position (Figure 7.4). It can, however, be active, for example forced breathing and coughing, where the expiratory muscles assist the elastic forces (resulting in a more rapid expiratory rate of flow and faster lung deflation). Compliance (demonstrated by the pressure volume curve) is a measure of the distensibility of the lungs or ease with which the lungs inflate (i.e. the reciprocal of elastance). It is measured as the change of volume in the lungs in response to a change in pressure. Normal lung and chest wall compliance is ~1L per kPa (100 mL per cmH2O). It can be seen in Figure 7.5 that lung compliance is lowered at high and low lung volumes. Hence, it is favourable for the patient to sit on the steep slope portion of the curve (it is easier to inflate a partially opened lung than a collapsed lung). The second group of opposing forces encountered in ventilation is the frictional forces. Impedance to air movement through the airways is called airways resistance. A small, but measurable, amount of work must be done to maintain the flow. Frictional forces are therefore dependent upon the speed with which air moves through the airway. Resistance is defined as the ratio of the pressure change responsible for air movement and the rate of flow. A normal value for resistance is around 2.5 cmH2O/L/sec. Factors affecting airways resistance include the size of the airway, its shape and calibre (Figure 7.5). Different diseases affect such properties, thus altering airway resistance, for example chronic obstructive pulmonary disease (COPD) is the most frequently encountered lung disease that increases airways resistance. With the presence of lung disease, the opposing forces of ventilation are increased. To sufficiently ventilate the lungs, larger patient efforts may be necessary to generate the pressure change needed to overcome the increased elastance or resistance (see Figure 7.6). Sustaining large inspiratory efforts may lead to excessive workloads and eventually respiratory fatigue and failure. Respiratory failure occurs when there is an imbalance of ventilatory requirements to neurocardiorespiratory capacity. It is a common medical condition that occurs when the lungs fail to oxygenate arterial blood adequately (type I respiratory or lung failure) and/or the muscle pump fails to prevent undue CO2 retention (type II ventilatory or pump failure). While no absolute diagnostic values for arterial oxygen (PaO2) and PaCO2 have been defined, values generally quoted are a PaO2 of less than 8.0 kPa and a PaCO2 of greater than 6.0 kPa (Roussos and Koutsoukou 2003). The mechanical disadvantage of a reduced FRC results from the consequential reduction of lung compliance and increased resistance, resulting in a greater work of breathing with a higher metabolic cost. The resultant clinical picture is that of rapid shallow breathing, which, in turn, further increases both oxygen consumption and carbon dioxide volume for excretion (Table 7.2). This is caused by failure of the respiratory pump (consisting of the respiratory muscles, chest wall, higher centres and nerves) where the amount of CO2 excreted is less than that produced by metabolism. With respiratory pump failure, even with normal lung pathology, the arterial PaCO2 is raised, with an inevitable fall in alveolar oxygen tension and hypoxaemia (alveolar hypoventilation). Hypoventilation results from a reduced respiratory effort, an inability to overcome resistive ventilatory forces or an inability to compensate for extra deadspace and/or CO2 production. The respiratory muscle pump is as vital as the heart, failing in the same way and for the same reasons, but a large respiratory reserve is present and function may be markedly impaired without ventilatory failure accompaniment (Table 7.3). • a reduction in neuromuscular competence resulting in the inability to generate adequate respiratory muscle force, for example reduced central drive, spinal injury, trauma, muscle disease (Figure 7.7); • an increase in respiratory workload caused by raised ventilatory impedance, for example obstructive and restrictive lung defects. Table 7.4 Causes of acute alveolar hypoventilation (Reproduced from Roussos and Koutsoukou (2003), with permission.)
Adult spontaneous and conventional mechanical ventilation
Introduction
Spontaneous ventilation
The respiratory muscles
Characteristics
Type I
Type IIa
Type IIb
Contractile
Contraction velocity
Slow
Fast
Fast
Myosin adenosine triphosphatase
Slow
Fast
Fast
Twitch duration
Long
Short
Short
Calcium ion sequestration
Slow
Rapid
Rapid
Metabolic
Capillaries
Abundant
Intermediate
Sparse
Glycolitic capacity
Low
Intermediate
High
Oxidative capacity
High
High
Low
Glycogen content
Low
Intermediate
High
Myoglobin content
High
Intermediate
Low
Fibre diameter
Small
Intermediate
Large
Motor unit size
Small
Intermediate
Large
Recruitment order
Early
Intermediate
Late
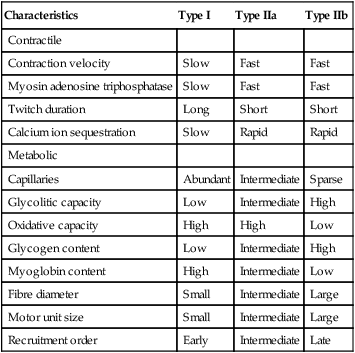
Respiratory mechanics and airflow
The opposing forces to ventilation
Compliance
The frictional forces
Respiratory failure
Acute hypoxaemic (type I) respiratory failure
Ventilatory (type II) respiratory failure
Pathways to respiratory failure
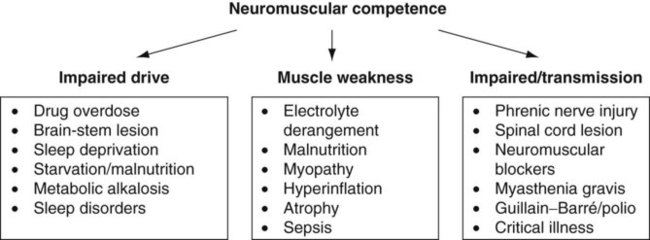

![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Musculoskeletal Key
Fastest Musculoskeletal Insight Engine