CHAPTER 11 Adjuvant Analgesics for Radicular Pain
PATHOPHYSIOLOGIC BACKGROUND OF RADICULAR PAIN
The pathophysiology of radicular pain remains incompletely understood. It is known that radicular pain is caused by disorders of the nerve root proximal to the dorsal root ganglion (DRG) or at the DRG itself. Proposed mechanisms of radicular pain include: (1) local neuropathic pain originating from lesions of nociceptive sprouts within the degenerated disc, (2) mechanical neuropathic root pain originating from mechanical compression of the nerve root, or (3) inflammatory neuropathic root pain derived from the action of inflammatory mediators originating from the degenerative disc.1 Traditionally, it has been thought that radicular pain arises from mechanical nerve root compression by herniated discs or osteophytes. However, increasing evidence suggests that radicular pain may involve chemical irritation or damage mediated by various agents released from the degenerated nucleus pulposus of herniated discs. These agents may include phospholipase A2,2 TNF-α,3 interleukin (IL)-8,4 IL-6, IL-1β,5 and nitric oxide.6 Chemical damage may lead to a state of hyperexcitability in the injured DRG or nerve root, a phenomenon called peripheral sensitization. The net result is ectopic neuronal firing7 transmitted via sensory neuron-specific, voltage-gated sodium channels. Ectopic neuronal firing also activates N-type voltage-gated calcium channels, permitting calcium influx and the promotion of excitation. As a result, dorsal horn neurons or central neurons innervated by the injured DRG or nerve root undergo dramatic functional changes, including a state of hyperexcitability termed central sensitization.8 Normally, these sensitization events spontaneously resolve as tissue healing occurs and inflammation subsides. Yet, when the primary afferent function is persistently altered by chemical or mechanical injury to the nerve roots, these processes may be highly resistant to treatment.9 Early antiinflammatory intervention (e.g. steroid injection) may reduce nerve root damage, thereby lessening the chance of developing chronic radicular pain. Indeed, this outcome may be the result of treating the peripheral disease, which in turn may eliminate the triggering of central sensitization.10
USE OF ANTIEPILEPTIC DRUGS IN CHRONIC RADICULAR PAIN
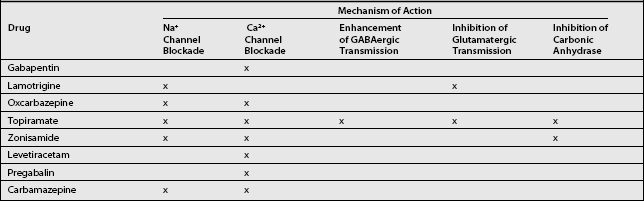
Current first-line analgesic therapies for chronic radicular pain rely mostly on nonsteroidal antiinflammatory drugs (NSAIDs) known to relieve nociceptive pain only. Since the pathophysiology of neuropathic pain and epilepsy are similar, the use of antiepileptic drugs (AEDs) in the treatment of neuropathic pain is also an attractive option.11 Mechanisms of central sensitization and ectopic neuronal firing are common to both epilepsy12 and neuropathic pain.13 Given the similarities between these two disorders, it is reasonable to speculate that the mechanisms of action that may be responsible for the therapeutic efficacy of the newer AEDs in the treatment of epilepsy may also prove beneficial in the treatment of neuropathic pain.14 Several mechanisms of action shared by the newer AEDs relate directly to the pathophysiology of neuropathic pain. Any or all of these actions may account for the effectiveness of these medications: (1) sodium channel blockade,15 (2) calcium channel blockade,15 (3) enhancement of GABAergic transmission,16 (4) inhibition of glutamatergic transmission,16 and (5) inhibition of carbonic anhydrase17 (Table 11.1). The mechanisms of chronic radicular pain may differ somewhat from those of peripheral neuropathic pain, resulting in varying of response to medication.18
Interestingly, a recent survey in the US19 indicated that AEDs are the third most commonly used class of medications for treating radiculopathy, after NSAIDs and opioids. Clinical trials demonstrating the efficacy of AEDs in this indication would be highly valuable, since therapeutic modalities such as AEDs could be a useful alternative for patients who do not respond to NSAIDs.1
NEWER ANTIEPILEPTIC DRUGS
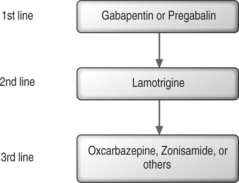
Recommendations for first-line AEDs are based on the positive results from multiple randomized, controlled trials. First-line medications for neuropathic pain include gabapentin, tricyclic antidepressants (TCAs), and tramadol hydrochloride (Fig. 11.1).9
Gabapentin: first-line antiepileptic drugs for chronic radicular pain
Gabapentin is an AED with an unknown mechanism of action apparently dissimilar to that of other antiepileptic agents. This agent also possesses desirable pharmacokinetics: gabapentin is not protein bound and is not metabolized, and does not induce liver enzymes, diminishing the likelihood of interactions with other antiepileptic agents and with other drugs such as oral contraceptives. Although gabapentin is a structural analog of the neurotransmitter gamma-aminobutyric acid (GABA), which does not cross the blood–brain barrier, gabapentin does penetrate into the CNS.20 Evidence suggests that its mechanism of action most likely involves complex synergy between increased GABA synthesis, non-NMDA receptor anatagonism, and binding to the α2δ subunit of voltage-dependent calcium channels. The latter action inhibits the release of excitatory neurotransmitters.21
The most important action of gabapentin appears to be its binding to the α2δ subunit of voltage-dependent calcium channels (see Table 11.1).22 These binding sites are located in the spinal cord, with particularly high density in the superficial laminae of the dorsal horn. The action of gabapentin at these sites may inhibit the release of excitatory neurotransmitters and reduce glutamate availability at NMDA and non-NMDA receptors.23
In chronic radiculopathy, mechanical and chemical nociceptive stimuli lead to the production of ectopic impulses. These ectopic discharges are seen along the length of the nerve root fiber. Ectopic discharges can provide sustained afferent input to the spinal cord from a damaged root or DRG. Gabapentin may inhibit these discharges at the level of the nerve root, DRG,24 spinal cord,21,23 or brain.20
Dosing and titration
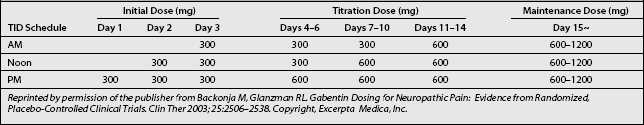
Titration and dosing schedules (Table 11.2) may potentially be efficacious in treating radicular pain and promoting tolerability, particularly in elderly patients, who make up a large proportion of radicular pain patients.25
Initiation
Based on the schedule of treatment initiation used in study protocols, it is reasonable to start gabapentin at 300 mg once daily on day 1, 300 mg twice daily on day 2, and 300 mg thrice daily on day 3 (see Table 11.2). This stepwise escalation was well tolerated in clinical studies and has the advantages of simplicity and rapidity in reaching the goal dosage of 900 mg/day. These factors may contribute to patient compliance. Although infrequent, in cases of intolerance gabapentin should be initiated at lower dosages, such as 100 mg in a single dose at bed time, then titrated daily by 100 mg three times.25
Titration and maintenance
In the reviewed trials, clinically relevant improvements were noted at week 2, during which patients received gabapentin 1800 mg/day. Thereafter, dosages were increased from 1800 to 3600 mg/day as tolerated to achieve better efficacy. In many patients, further dose escalations up to 3600 mg/day may be necessary to reach individualized maximally effective doses that can be maintained without compromising tolerability. Table 11.2 provides a possible schema for stepwise escalations in gabapentin dosing. Dosage increases can be simplified by the availability of 600 and 800 mg gabapentin tablets.
Although drowsiness may occur during the initial titration period, the overall low rate of serious side effects associated with gabapentin treatment in clinical trials indicate that rapid dose escalations are probably safe.20,26,27 In most cases, drowsiness generally resolves within 7–14 days from the initiation of treatment. Contrary to previously published reports, however, some patients may continue to complain of drowsiness of moderate severity even at a daily dose of 900 mg. Based on study findings and the author’s clinical experience, some adverse events may be due to the dose titration process itself. Therefore, patient education about the transient nature of such adverse events may allow early achievement of effective doses and improve patient satisfaction.25 To minimize the problem of persistent drowsiness or other side effects, one can increase the intervals between dosage escalations, based on each patient’s tolerance.
Maximal effective dose
On the basis of reviewed trials, doses of up to 3600 mg/day may be used when required and tolerated, and can be achieved by week 4 of treatment. In an animal model, gabapentin was shown to be more efficacious at lower doses in treating radicular pain than peripheral neuropathic pain.18 However, these findings need to be investigated further in future studies.
Combination treatment with tricyclic antidepressants, and tramadol
Gabapentin, tricyclic antidepressants (TCAs), and tramadol hydrochloride have been used as first-line medications for neuropathic pain. It is common for patients to have a partial response to these medications, and in these cases combination treatment should be considered. No studies have systematically examined the efficacy of various combinations of these three medications, as compared with monotherapy. Despite the lack of controlled data, combinations of two or more of these first-line medications can be recommended when patients have a partial response to monotherapy or at the beginning of treatment. The disadvantage of combination therapy is the difficulty in identifying which medication is responsible for any adverse effects.9
Clinical reports on chronic radiculopathy
Although several published, double-blind, placebo-controlled, randomized clinical trials of gabapentin in the treatment of chronic neuropathic pain have been published,27–30 there are only a few clinical reports designed to study the effect of gabapentin on pathological processes involving the dorsal root ganglion and proximal nerve root. These processes include chronic radiculopathy, arachnoiditis, and epidural fibrosis.
Chronic radiculopathy
A unique randomized, placebo-controlled study evaluated the efficacy of gabapentin monotherapy in patients with chronic radiculopathy over 8 weeks. The results showed significant improvement in the following: pain at rest, straight-leg raise test (SLR), and limitation of spinal flexion. The gabapentin group was treated with doses ranging from a total of 900 mg/day to 3600 mg/day divided into three doses.31
Arachnoiditis
In one open-label study, three patients with arachnoiditis were treated with gabapentin at a maximum of 2700 mg/day. One patient discontinued the study because of adverse effects, and the other two had moderate improvement in their pain.32
Epidural fibrosis
There was one case report in which two patients were treated for radicular pain resulting from epidural fibrosis caused by failed back surgery syndrome. It was reported that functional status improved markedly and pain was significantly diminished with gabapentin regimens of 1500 mg and 2100 mg/day.33
Adverse effects
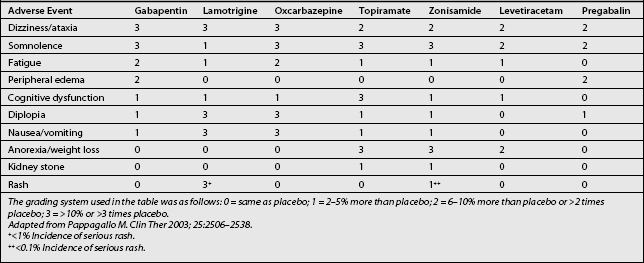
The adverse effects of gabapentin include somnolence, fatigue, dizziness, and, less commonly, mild peripheral edema and gastrointestinal symptoms (Table 11.3). The incidence of mild adverse events has been reported in up to 35–75% of gabapentin recipients, seemingly in a dose-dependent manner.20,26,27 All of these effects require monitoring and dosage adjustment, but usually not discontinuation of the drug. In elderly patients in particular, gabapentin may cause gait and balance problems, as well as cognitive impairment. In addition, dosage adjustment is necessary in patients with renal insufficiency. However, generally excellent tolerability and safety of gabapentin distinguish it from most other oral medications used for the treatment of chronic neuropathic pain. In effect, it is often the first choice for treating many types of neuropathic pain.34 Gabapentin is absorbed orally without interference by food, and it reaches peak serum concentrations after 2–3 hours. No blood monitoring is required with gabapentin therapy because of the absence of toxic effects.20
Lamotrigine
Lamotrigine is a novel AED with at least two antinociceptive properties: it stabilizes the neural membrane through blocking the activation of voltage-sensitive sodium channels, and it inhibits the presynaptic release of glutamate.15 Abnormal neural firing is a principle cause of nerve injury-induced pain,7 and glutamate plays a key role in dorsal horn spinal hyperexcitability by acting at the NMDA receptor (see Table 11.1),35 providing a strong rationale for the use of lamotrigine in the treatment of radicular pain.
Dosing and titration
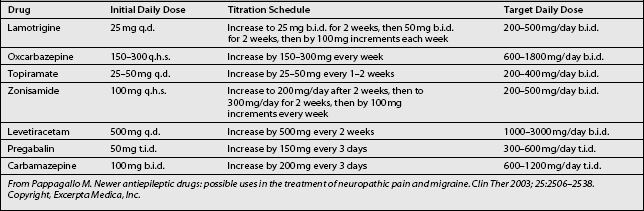
Titration (Table 11.4) is initiated at a dose of 25 mg daily for 2 weeks, increasing to 50 mg/day for 2 weeks. Further increases are by 50 mg b.i.d. for 2 weeks, then by 100 mg/day b.i.d. every week,11,36 until the desired daily dose is achieved. The regimen of gradual dose increments is chosen to avoid the occurrence of adverse drug reactions.20 The analgesic effects of lamotrigine become more profound after prolonged treatment with a steady dose.36
Clinical reports on chronic radiculopathy
Lamotrigine in doses higher than 200–400 mg daily has demonstrated efficacy in relieving pain in patients with trigeminal neuralgia, complex regional pain syndrome type I, chronic neuropathic pain syndrome, painful HIV neuropathy, central post-stroke pain, and incomplete spinal cord lesions.9
A unique open-label study investigated the effect of lamotrigine on chronic radicular pain, and showed that spontaneous pain, pain associated with the straight-leg-raising test), and pain associated with bending the affected side reached a statistically significant level of improvement only at a 400 mg dose. These results suggest that lamotrigine is a potentially effective treatment for painful lumbar radiculopathy, and that it is likely to act in a dose- and plasma concentration-dependent fashion.36 Because of the slow and careful titration required and risk of both severe rash and Stevens-Johnson syndrome associated with its use, lamotrigine should, however, only be considered in cases of chronic radicular pain refractory to gabapentin.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree