Chapter 23 Adenosine-Sensitive (Outflow Tract) Ventricular Tachycardia
Classification
Ventricular tachycardia (VT) is usually associated with structural heart disease, with coronary artery disease and cardiomyopathy (CMP) being the most common causes. However, about 10% of patients who present with VT have no obvious structural heart disease (idiopathic VT).1 Absence of structural heart disease is usually suggested if the electrocardiogram (ECG) (except in Brugada syndrome and long QT syndrome), echocardiogram, and coronary arteriogram collectively are normal. Nevertheless, magnetic resonance (MR) imaging may demonstrate mild structural abnormalities and subtle areas of diminished wall motion in some patients with idiopathic VT, even if all other test results are normal. In addition, focal dysautonomia in the form of localized sympathetic denervation has been reported in patients with VT and no other obvious structural heart disease. Of note, idiopathic VT occasionally occurs in patients with structural heart disease, in whom the structural heart disease is not related to the VT. Furthermore, frequent or incessant idiopathic VT can be a cause of tachycardia-induced CMP.2
Several distinct types of idiopathic VT have been recognized and classified with respect to the origin of VT (right ventricle [RV] versus left ventricle [LV]), VT morphology (left bundle branch block [LBBB] versus right bundle branch block [RBBB] pattern), response to exercise testing, response to pharmacological agents (adenosine-sensitive versus verapamil-sensitive versus propranolol-sensitive VT), and behavior of VT (repetitive salvos versus sustained).3
Pathophysiology
Mechanism of Adenosine-Sensitive Ventricular Tachycardia
Most forms of outflow tract VTs are adenosine-sensitive and are thought to be caused by catecholamine-induced, cyclic adenosine monophosphate (cAMP)–mediated delayed afterdepolarizations (DADs) and triggered activity, which is supported by several tachycardia features (see Chap. 4).4 Heart rate acceleration facilitates VT initiation. This can be achieved by programmed stimulation, rapid pacing from either the ventricle or the atrium, or infusion of a catecholamine alone or during concurrent rapid pacing. Additionally, termination of the VT is dependent on direct blockade of the dihydropyridine receptor by calcium channel blockers or by agents or maneuvers that lower cAMP levels (e.g., by activation of the M2 muscarinic receptor with edrophonium or vagal maneuvers, inhibition of the beta-adrenergic receptor with beta blockers, or activation of the A1 adenosine receptor with adenosine). Furthermore, a direct relationship exists between the coupling interval of the initiating ventricular extrastimulus (VES) or ventricular pacing cycle length (CL) and the coupling interval of the first VT beat. Additionally, VT initiation is CL-dependent; pacing CLs longer or shorter than a critical CL window fail to induce VT. This critical window can shift with changing autonomic tone.1,5,6
Types of Adenosine-Sensitive Ventricular Tachycardia
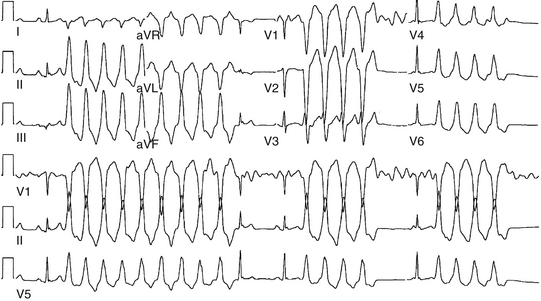
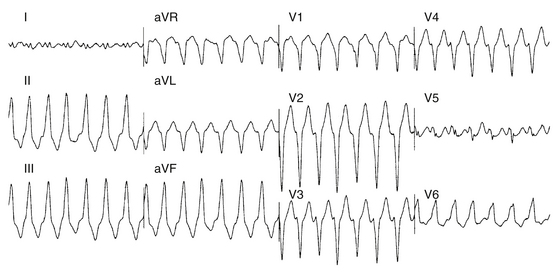
Approximately 90% of idiopathic VTs are caused by one of two phenotypic forms of adenosine-sensitive VT. Repetitive monomorphic VT is the most common form (60% to 90%) and is characterized by frequent premature ventricular complexes (PVCs), couplets, and salvos of nonsustained VT, interrupted by brief periods of normal sinus rhythm (NSR; Fig. 23-1). This form of VT usually occurs at rest or following a period of exercise, and typically decreases during exercise, but can be incessant.1 On the other hand, paroxysmal exercise-induced VT is characterized by sustained episodes of VT precipitated by exercise or emotional stress, separated by long intervals of NSR with infrequent PVCs (Fig. 23-2).1 Evidence has suggested that both types represent polar ends of the spectrum of idiopathic VT caused by cAMP-mediated triggered activity, and there is considerable overlap between the two types. Furthermore, this subtype classification, although useful, is not necessarily precise and depends on the means and duration of rhythm recordings. Patients are typically categorized based on their presenting or index arrhythmia. Prolonged telemetry and long-term ambulatory ECG recordings have demonstrated that most patients with one subtype of outflow tract VT show evidence for at least one other subtype with an identical morphology. Almost all patients with nonsustained VT have high-density repetitive runs and frequent PVCs. In patients who present with repetitive PVCs, nonsustained VT can also be observed in approximately 70%; however, only 20% of these patients develop runs of more than five beats.7
Anatomical Considerations
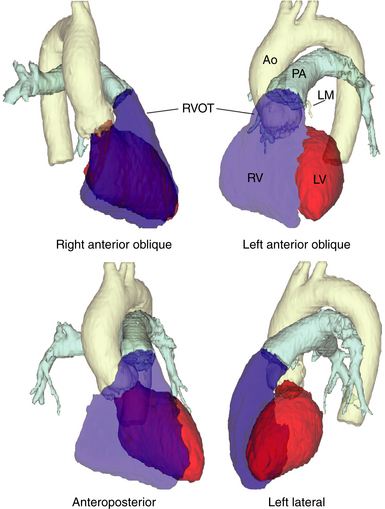
Adenosine-sensitive idiopathic VT usually arises from outflow tracts (most frequently from the RV outflow tract [RVOT]). Other variants of outflow tract VT (with similar underlying electrophysiological [EP] mechanism) include ventricular arrhythmias arising from the aortic cusps, pulmonary artery, mitral or tricuspid inflow tracts, papillary muscles, and epicardial foci in close proximity to the coronary venous system.3 Understanding the unique and complex anatomical relationships of the outflow tracts is critical for analyzing the ECG and mapping findings during outflow tract VT as well as for safe catheter maneuvering and ablation.
Right Ventricular Outflow Tract
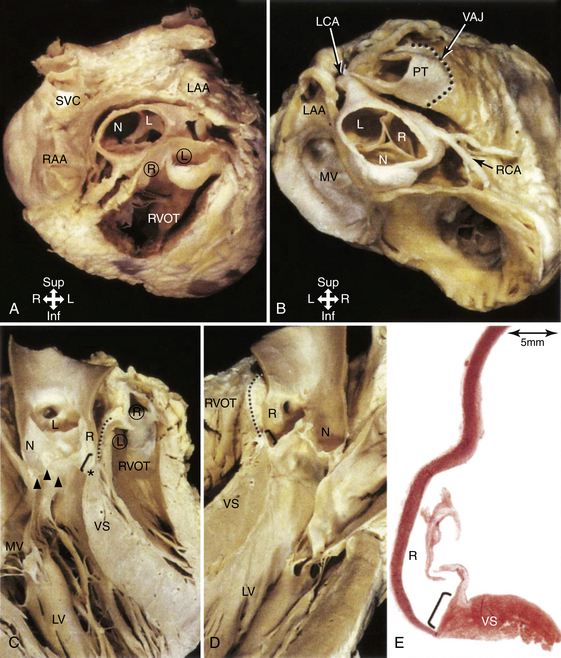
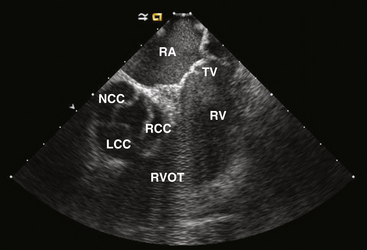
The RVOT is the tubelike portion of the RV cavity above the supraventricular crest, and is defined superiorly by the pulmonic valve and inferiorly by the RV inflow tract and the top of the tricuspid annulus (the region of the His bundle [HB] and proximal right bundle branch). The lateral aspect of the RVOT region is the RV free wall. The RVOT passes cephalad in a posterior and slightly leftward direction. The medial aspect is formed by the anterior interventricular septum at the base of the RVOT and RV musculature opposite to the anterior LV outflow region (LVOT) (as a cephalad continuation of the interventricular septum) and the root of the aorta (immediately adjacent to the right coronary cusp) at the region just inferior to the pulmonic valve (Fig. 23-3).8
Although the medial aspect of the RVOT is frequently referred to as the “septal” wall, it should be noted that the outflow tract per se is not part of the interventricular septum, and the septum is a component only of the most proximal part of the RVOT at the branch point of the septomarginal trabeculation. Above this area, the RVOT curves to pass anterior and cephalad to the LVOT, and, therefore, any perforation in the septal part is more likely to go outside the heart than into the LV (Fig. 23-4).
From the coronal view above the pulmonic valve, the RVOT region is seen wrapping around the LVOT and the root of the aorta and extending leftward (see Fig. 23-3). Whereas the inflow portion of the RV lies to the right and anterior to the inflow portion of the LV, the RVOT courses anterior to the LVOT such that the distal RVOT and pulmonic valve are located to the left side of the body in relationship to the aortic valve and the distal LVOT. The pulmonic valve is typically placed approximately 5 to 10 mm cephalad and to the left of the aortic valve such that the supravalvular portion of the aorta lies in immediate proximity to the portions of the pulmonic valve; immediately anterior to the aortic valve is the posterior muscular infundibular portion of the RVOT.8,9
The top of the RVOT can be convex or crescent-shaped, with the posteromedial region directed rightward and the anterolateral region directed leftward. The anteromedial aspect of the RVOT actually is located in close proximity to the LV epicardium, adjacent to the anterior interventricular vein and in proximity to the left anterior descending coronary artery. The aortic valve cusps sit squarely within the crescent-shaped posterior region of the RVOT and are inferior to the pulmonic valve (see Fig. 23-3). The most posterior aspect of the RVOT is adjacent to the region of the right coronary cusp, and the posteromedial (leftward) surface is adjacent to the anterior margin of the right coronary cusp or the medial aspect of the left coronary cusp.10 The thickness of the RVOT wall is variable, ranging from approximately 3 to 6 mm, and is thinnest in the rightward, anterior, and subpulmonic valve portions, and thickest in the posterior infundibular part that is adherent to the anterior LVOT as a cephalad continuation of the interventricular septum.8
Left Ventricular Outflow Region
Unlike the RV, the inflow and outflow tracts of the LV are at an acute angle to one another. The central location of the aortic valve places the LVOT between the mitral valve and the ventricular septum. In turn, approximately half of the aortic outlet is muscular and the other half (being the area of valvular continuity between the mitral and aortic valves) is fibrous. The curvature of the ventricular septum continuing into the free wall forms the anterosuperior wall of the LVOT. It is the deep anterior (aortic) leaflet of the mitral valve that forms the aortic-mitral curtain. The extremities of the fibrous continuity are the left and right fibrous trigones, the right trigone forming the central fibrous body. The LVOT passes underneath the RVOT in a rightward and cephalad direction pointing toward the right shoulder (see Fig. 23-4).11
Aortic Cusps
The aortic root is defined as the interface between the LV and the ascending aorta, extending from the sinotubular junction in the aorta to the basal valvular leaflets with their attachment within the LV (see Fig. 23-3). Approximately two-thirds of the circumference of the lower part of the aortic root is connected to the muscular ventricular septum, with the remaining one-third in fibrous continuity with the aortic leaflet of the mitral valve. Its components are the sinuses of Valsalva, the fibrous interleaflet triangles, and the valvular leaflets themselves. The aortic valve is composed of three symmetric, semilunar-shaped cusps. The recess of each cusp is called the “sinus of Valsalva.” The aortic (coronary) cusps are firmly anchored to the fibrous skeleton within the root of the aorta. A circular ridge on the innermost aspect of the aortic wall, at the upper margin of each sinus, is the sinotubular ridge—the junction of the sinuses and the aorta. The aortic cusps are named according to their orientation in the body—left and right (both facing the pulmonic valve anteriorly) and posterior. The left aortic sinus gives rise to the left coronary artery, and the right aortic sinus gives rise to the right coronary artery. Usually, no vessels arise from the posterior aortic sinus, which is therefore known as the noncoronary sinus. Three equally spaced sites of minimal tethering within the aortic root mark the junctions of the sinuses of Valsalva. Each sinus is associated with a leaflet of the aortic valve, whereas the junctions between the adjacent sinuses are aligned with the commissures between the aortic valve leaflets.12–14
The aortic valve is the cardiac centerpiece; it lies in contact or continuity with all four cardiac chambers and shares important proximate relationships with each of the other cardiac valves (Video 20  ). It comes into contact with the right atrium (RA), left atrium (LA), interatrial septum, RVOT, mitral valve (aortomitral continuity), pulmonic valve, tricuspid valve, and conduction system (see Fig. 23-3).8,9
). It comes into contact with the right atrium (RA), left atrium (LA), interatrial septum, RVOT, mitral valve (aortomitral continuity), pulmonic valve, tricuspid valve, and conduction system (see Fig. 23-3).8,9
The aortic root is inferior, posterior, and somewhat rightward compared with the RVOT. As noted, the posterior subpulmonic valve portions of the RVOT are immediately anterior and more proximally continuous with the anterior myocardial subaortic LVOT and more distally to the right coronary cusp. The lateral and more distal portions of the left coronary cusp lie immediately subjacent to the peripulmonic valve portions of the RVOT. In some instances, the supravalvular myocardial fibers of one outflow tract may be continuous with the other outflow tract’s infravalvular myocardium, thus forming a functional syncytium.8
The left coronary cusp lies to the left of the right coronary cusp and is related to the posterior wall of the RVOT (Fig. 23-5). The commissure between the right and left coronary cusps is just posterior to the distal RVOT and is close but caudal to the posterior pulmonic valve annulus. More leftward and posteriorly, the left coronary cusp lies in continuity with the anterior leaflet of the mitral valve (aortomitral continuity). Adjacent to this site lies the peripulmonic valve myocardium and, more laterally, the posterior lobe of the LA appendage (when present).8,9
The right coronary cusp lies immediately posterior to the relatively thick posterior infundibular portion of the RVOT. Caudally, there is continuity with the anterior LVOT, and at the level of valve insertion there is either physical continuity or very close proximity between myocardium that extends above this valve cusp, the LVOT, and the posterior RVOT. The posterior part of the right coronary cusp is adjacent to the central fibrous body, which carries within it the penetrating portion of the HB. Anteriorly, the right coronary cusp is related to the bifurcating atrioventricular (AV) bundle and the origin of the left bundle branch. The right coronary cusp does not have a direct relationship with either atrium, but lateral to the commissure with the noncoronary cusp lies the RA appendage, trunk of the right coronary artery, and a variable amount of fat.8,9
The noncoronary cusp is the most posterior of the aortic cusps, and lies immediately anterior to the interatrial septum and superior to the central fibrous body, and has the RA and LA as the posterior right and left relations, respectively (see Fig. 23-5). In fact, atrial tachycardias have reportedly been ablated from within the noncoronary cusp because of its close relationship to the atria. Caudally, like the other cusps, the noncoronary cusp is in continuity with the LVOT myocardium. Other than this site, however, it has no anatomical relationship with any other ventricular myocardium. The triangle between the noncoronary and the right coronary sinuses incorporates within it the membranous part of the septum (the location of the penetrating HB). This fibrous part of the septum is crossed on its right side by the hinge of the tricuspid valve, which divides the septum into atrioventricular and interventricular components.8,9,12,13
Because of the semilunar nature of the attachments of the aortic valvular leaflets, there are three triangular extensions of the LVOT that reach to the level of the sinotubular junction. These triangles, however, are formed not of ventricular myocardium but of the thinned fibrous walls of the aorta between the expanded sinuses of Valsalva.14
For the greater part, the aortic sinuses are made up of the wall of the aorta. However, sleeves of ventricular myocardium extend beyond the aortic valve attachments for variable distances (analogous to atrial myocardial extensions in the pulmonary veins). Whereas the right coronary cusp and the anterior portions of the left coronary cusp frequently exhibit these myocardial sleeves, the posterior portions of the left coronary cusp and the noncoronary cusp, particularly in relation to the fibrous continuity with the anterior leaflet of the mitral valve (the aortomitral continuity), are exclusively fibrous and usually devoid of myocardium.8,9,15 The noncoronary cusp at its junction of the right coronary cusp may have sleeves of ventricular myocardium and, at present, it is not clearly known whether myocardial extensions into the noncoronary cusp represent atrial or ventricular myocardium.8
Idiopathic VT can originate from the right or, more commonly, left coronary cusp, as well as from the junction of the left and right coronary cusps. The substrate of this VT likely originates from the strands of ventricular myocardium present at the bases of those cusps. In contrast, the base of the noncoronary cusp is composed of fibrous tissue and, thus, is an extremely rare site of origin of VT.16,17 However, the nature of the actual substrate being ablated has not been clearly defined. For example, the ablation electrode placed in the depth of the right coronary cusp may be mapping and ablating a focus arising from the supravalvular extension into the cusp, LVOT myocardium, or a deeper posterior RVOT myocardium.9
Pulmonary Artery
The pulmonary sinuses are not as prominent as the aortic sinuses. Nevertheless, owing to the semilunar configuration of the valvular leaflets, the hinge line of each leaflet crosses the ventriculoarterial junction at two points. Consequently, there are always small segments of myocardium of the infundibulum at the nadirs of the three sinuses. Between adjacent sinuses, the wall comprises small triangles of fibrous tissue that become incorporated into the RV when the valve closes. On the epicardial aspect, the ventriculoarterial junction is not always a sharply defined line. Extensions of ventricular myocardium into the adventitia occur in approximately 20% of individuals and have been traced to a maximal distance of 6 mm beyond the junction.11
As noted, the pulmonic and aortic valves are not at the same level (see Fig. 23-3). The pulmonic valve, the most superiorly situated of the cardiac valves, lies at the level corresponding to the third left costal cartilage at its junction with the sternum. The transverse plane of the aortic valve slopes inferiorly, away from the plane of the pulmonic valve, such that the orifice of the aortic valve faces rightward at an angle of at least 45 degrees from the median plane.11
Because of its anterior and leftward location, only the posterior and rightward parts of the pulmonary artery have important relations with other cardiac structures. Of the three cusps of the pulmonic valve, the septal (right) pulmonic cusp lies at variable distances from and sometimes adjacent to the distal portions of the RA appendage. The left pulmonic cusp, being the most superficial, lies immediately beneath the pericardium and has no other cardiac structures related to it. The posterior pulmonary cusp externally lies in the proximal portion of the left main coronary artery and the distal portions in the LA appendage. The supravalvular portion of the aorta lies close to and in some cases adjacent to the junction and surrounding parts of the right and posterior pulmonic cusps.8,9
The pulmonic valve and supravalvular portion of the pulmonary artery are well-established locations of origin for ventricular arrhythmias. As noted, ventricular myocardial sleeves extend above the semilunar valves for a variable distance (a few millimeters and up to more than 2 centimeters). Whereas the myocardial sleeves typically extend circumferentially around the pulmonic valve between and above all three cusps just above the annulus, more distally the extension is patchy and generally asymmetrical. Myocardial extensions also occur in the intercuspal clefts in addition to within the cusps.8
Clinical Considerations
Epidemiology
Approximately 60% to 80% of idiopathic VTs arise from the RV (most commonly the RVOT). RVOT VT comprises 10% of all VTs referred to an electrophysiologist. Age at presentation is usually 30 to 50 years (range, 6 to 80 years). Women are more commonly affected.1,5
Clinical Presentation
Very frequent idiopathic nonsustained VT, PVCs, or both can precipitate a reversible form of LV systolic dysfunction, similar to tachycardia-induced dilated CMP. The relationship between the LV dysfunction and VT may not be initially recognized, and those patients can present with heart failure symptoms and become diagnosed with nonischemic dilated CMP and may even undergo prophylactic implantation of a defibrillator, which unfortunately often results in delivery of inappropriate shocks triggered by frequent nonsustained episodes of idiopathic VT.2 Interpolation of the PVCs appears to predict an increased risk of PVC-induced CMP.18 Notably, in a recent report, 20% of patients with PVC-induced CMP had the PVC focus in one of the aortic cusps.19
Initial Evaluation
The diagnosis of ARVD should be carefully considered. Signal-averaged ECG, MR imaging of the RV, RV biopsy, and RV angiography are all unremarkable in idiopathic RVOT VT and help exclude ARVD. An invasive EP study is usually not necessary to establish a diagnosis, although it can occasionally help exclude other forms of tachyarrhythmias (see later).2
It is important to recognize that, in some patients, idiopathic VT can coexist with structural heart disease, including CMP, in which setting the VT is not related to the cardiac disease. On the other hand, very frequent idiopathic nonsustained VT, PVCs, or both can precipitate dilated CMP. The responsible ectopy can arise from any ventricular site, but the RVOT, being the most common type of idiopathic ventricular ectopy, is more commonly associated with ectopy-related CMP. Therefore, in patients who present with a dilated CMP of unclear etiology and who have frequent PVCs or nonsustained VT, it is important to assess the contribution of PVCs or VT to LV systolic dysfunction. A reversible form of dilated nonischemic CMP precipitated by idiopathic VT should be suspected when very frequent PVCs, nonsustained VT, or both are observed on a 24-hour Holter recording, especially when the QRS morphology is monomorphic and is suggestive of origin from the outflow tract. A PVC burden of more than 20% is present in most of those patients with tachycardia-induced CMP, but some patients have as few as 5% PVCs. In a recent report, a cutoff PVC burden of more than 24% was strongly associated with the presence of CMP. However, this cutoff value failed to identify every patient at risk of CMP, and for individual patients, the critical PVC burden can be lower. A cutoff PVC burden of more than 16% would result in a sensitivity of 90% for CMP, but the specificity would be reduced to 58%.20 Although pharmacological suppression of the PVCs, such as by amiodarone therapy, may help evaluate the relationship between the arrhythmia and CMP, the usefulness of this approach and duration of therapy required have not been defined. Often, elimination of the PVCs by catheter ablation results in improvement and resolution of LV dysfunction within a few months. Therefore, catheter ablation of the PVCs is appropriate prior to implantable cardioverter-defibrillator (ICD) implantation for primary prevention of SCD; successful elimination of the PVCs may result in improvement in LV function such that the patient no longer qualifies for an ICD.2,21
Principles of Management
Chronic Management
Long-term treatment options for outflow tract VT include medical therapy and catheter ablation. Medical therapy may be indicated in patients with mild to moderate symptoms. For patients with symptomatic, drug-refractory VT or those who are drug intolerant or who do not desire long-term drug therapy, catheter ablation is the treatment of choice. Catheter ablation is also recommended for patients with frequent PVCs or nonsustained VT when they are presumed to cause LV dysfunction, even in otherwise asymptomatic patients.2
Electrocardiographic Features
Surface Electrocardiogram
Ecg During Ventricular Tachycardia
Repetitive monomorphic VT is characterized by frequent PVCs, couplets, and salvos of nonsustained VT, interrupted by brief periods of NSR (see Fig. 23-1). Paroxysmal exercise-induced VT is characterized by sustained episodes of VT precipitated by exercise or emotional stress (see Fig. 23-2). Both types characteristically have an LBBB pattern with a right inferior (more common) or left inferior axis. The tachycardia rate is frequently rapid (CL < 300 milliseconds), but can be highly variable. A single morphology for the VT or PVCs is characteristic.1,6
Ambulatory Monitoring
Several VT characteristics can be observed on ambulatory monitoring recordings. Ventricular ectopy typically occurs at a critical range of heart rates (CL dependence). The coupling interval of the first PVC is relatively long (approximately 60% of the baseline sinus CL). A positive correlation exists between the sinus rate preceding the VT and the VT duration. Additionally, the VT occurs in clusters, and is most prevalent on waking and during the morning and later afternoon hours. The VT is extremely sensitive to autonomic influences, resulting in poor day-to-day reproducibility.1,6
Electrocardiographic Localization of Outflow Tract Ventricular Tachycardia
VTs originating from the RVOT typically display LBBB morphology with a precordial QRS transition (first precordial lead with R/S ratio >1) that begins no earlier than lead V3 and more typically occurs in lead V4. The frontal plane axis, precordial R/S transition, QRS width, and complexity of the QRS morphology in the inferior leads can more precisely indicate the origin of VT within the RVOT. Most RVOT VTs originate from the anterosuperior aspect of the leftward (“septal”) aspects, just under the pulmonic valve. These tachycardias produce a characteristic 12-lead ECG appearance with tall positive QRS complexes in leads II, III, and aVF and large negative complexes in leads aVR and aVL. The QRS morphology in lead I typically is multiphasic and has a net QRS vector of zero or is only modestly positive (see Fig. 23-2).
However, not all VTs with a QRS morphology of LBBB and inferior or normal axis can be ablated successfully from the RVOT. Some VTs originate in the subaortic LV (LVOT, 10% to 15% of adenosine-sensitive VTs), above the pulmonic valve (i.e., from muscle tracts in the pulmonary artery), and occasionally in the aortic root. Idiopathic RV VTs with a superior QRS axis generally originate in the body of the RV on the anterior free wall, or in the mid- and distal septum (Table 23-1).
TABLE 23-1 Estimation Indexes of Right Ventricular Outflow Tract Ventricular Tachycardia Origins by 12-Lead ECG*
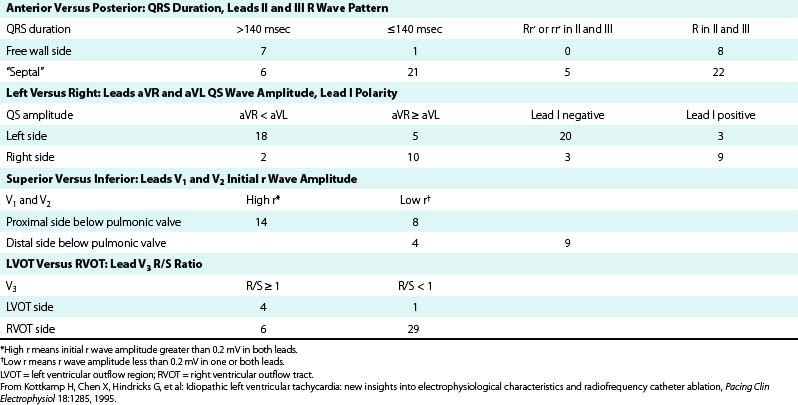
It is important to recognize that the prediction of the precise origin of outflow tract VT can still be challenging because of the close anatomical relationship of the different anatomical compartments of the outflow tract area. For example, an R/S transition zone in precordial lead V3 is common in patients with idiopathic outflow tract VT, with a prevalence of up to 58%. The prevalence of R/S transition in lead V3 in RVOT VT is not statistically different from VT originating outside the RVOT; therefore, the predictive value for this ECG criterion is low. Approximately 50% of outflow tract tachycardias with an R/S transition in V3 could be successfully ablated from the RVOT; however, one study has shown that a significant proportion of patients need different anatomical approaches for successful RF catheter ablation using up to six different anatomical accesses, including the LV, the aortic sinus of Valsalva, the coronary sinus (CS), the pulmonary artery, and the epicardium via a percutaneous pericardial puncture.22
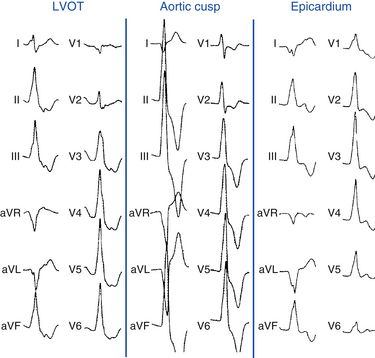
Right Ventricular Outflow Tract Versus Left Ventricular Outflow Region
The absence of an R wave in lead V1 and precordial transition zone in lead V4, V5, or V6 predicts an RVOT origin. On the other hand, the presence of an R wave in leads V1 and V2 and RS transition in leads V1 or V2 are characteristic of an LVOT origin (Figs. 23-6 and 23-7). Because of the continuity between the posterior RVOT and the anterior LVOT, a very similar R wave in lead V3 may be seen with arrhythmia that originates in either structure, and R/S transition in lead V3 is not specific. In the latter setting, a precordial R/S transition during VT that is earlier than that during NSR argues against an LVOT origin. Additionally, comparing the R wave amplitude divided by total QRS amplitude (i.e., R/[R + S]) in lead V2 during VT with that during NSR can help distinguish between LVOT and RVOT origins in patients with lead V3 precordial transition. A transition ratio (R/[R + S]VT ÷ R/[R + S]NSR) of at least 0.60 is highly suggestive of an LVOT origin.23 Furthermore, a QS complex in lead I is also suggestive of subaortic LV origin.8,22
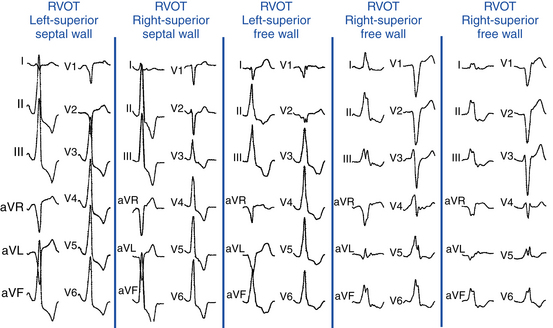
“Septal” Versus Free Wall Right Ventricular Outflow Tract
QRS duration less than 140 milliseconds, monophasic R wave without notching (i.e., no RR′ or Rr′) in leads II and III, and early precordial transition (by lead V4) suggest a septal origin. On the other hand, the triphasic RR′ or Rr′ waves in VT of free wall origin probably reflect the longer QRS duration and the phased excitation from the RV free wall to the LV (see Fig. 23-6).
Left (Anteromedial Attachment) Versus Right (Posterolateral Attachment) Side of The Right Ventricular Outflow Tract
In general, a QS complex in lead I is generated from sites at or near the anterior medial aspect of the RVOT (the most leftward portion of the RVOT in the supine anteroposterior orientation). As the site of origin moves rightward, on either the posterior or the anterior wall, R waves appear in lead I and become progressively dominant and the QRS axis becomes more leftward. Similarly, a QS amplitude in aVL greater than that in aVR suggests an origin in the left side of the RVOT; a QS amplitude in aVR greater than that in aVL suggests an origin in the right side (see Fig. 23-6).
Superior Versus Inferior Right Ventricular Outflow Tract
The R wave amplitude tends to be larger in leads V1 and V2 at superior and leftward sites; as the site of origin shifts to the right or inferiorly, there is a trend toward lower right precordial R wave amplitude and a shift in the precordial transition zone to the left. Furthermore, R wave amplitude in lead V2 or “r” wave amplitude in leads V1 and V2 greater than 0.2 mV suggests a superior origin. Additionally, the closer the origin to the pulmonic valve, the more rightward and inferior the axis (i.e., R wave taller in lead III than in lead II) because of the anatomical leftward location of the pulmonic valve and lead III being an inferior and rightward lead; the more posterior and inferior the origin, the more leftward the axis (see Fig. 23-6). Also, lead aVL (being a left-sided lead) becomes isoelectric or slightly positive as the site of origin moves inferiorly (close to the HB region), whereas aVR (being a right-sided lead) remains negative.8
Ventricular Tachycardias Arising above the Pulmonic Valve
Because of the superior and leftward location of these sites, VTs arising above the pulmonic valve are associated with a small but definite vector toward the right, resulting in a small initial R wave in lead V1. Additionally, suprapulmonic origins exhibit a strong right inferior frontal plane axis (a QS or rS wave in lead I, large R waves in II, III, aVF, and deep QS complexes in aVR and aVL, with the R wave being taller in lead III than in lead II and the S wave being deeper in aVL than in aVR). Those VTs also tend to display an R/S ratio in lead V2 and R wave amplitude in inferior leads that are significantly larger than those in RVOT VT.8,9,24 However, moderate overlap exists between VTs originating above the pulmonic valve and RVOT VTs, and because the RVOT is the exit site of VT arising from the pulmonary artery, discriminating between the two groups using ECG parameters can be difficult.25
Ventricular Tachycardias Arising from the Tricuspid Annulus
VTs arising from the tricuspid annulus demonstrate LBBB morphology and positive QRS polarity in leads I, V5, and V6. In contrast to VTs arising from the RVOT, no positive QRS polarities in any of the inferior leads characterize VTs arising from the tricuspid annulus. No negative component of the QRS complex is found in lead I, and the R wave magnitude in lead I is typically greater in VTs arising from the tricuspid annulus than those arising from the RVOT.26 Additionally, VTs arising from the tricuspid annulus have an rS or QS pattern in lead aVR, just as those arising from the RVOT; however, in lead aVL, a QS or rS pattern is rare (8%), and the QRS polarity in lead aVL is positive in almost all VTs arising from the annulus (89%), in contrast to those arising from the RVOT.26
Ventricular Tachycardias Arising from the Right Ventricular Papillary Muscles
RV papillary muscle VTs display LBBB morphology with a QS or rS pattern in lead V1 and a wider QRS complex and more prevalent notching than in RVOT VT. VTs arising from the anterior and posterior papillary muscles are frequently associated with a superior axis and a late R wave transition in the precordial leads (later than lead V4), whereas septal papillary muscle VTs more often display an earlier precordial transition (in lead V4 or earlier) and an inferior axis (due to the more basal insertion of the septal compared with the anterior and posterior papillary muscles). More than one PVC or VT morphology can be present in a significant proportion of patients with papillary muscle VTs.27 Compared with origins from the septum, VTs from the free wall exhibit longer QRS duration and deeper S waves in leads V2 and V3.28
Ventricular Tachycardias Arising from the Left Ventricular Papillary Muscles
VT can also arise from posterior or anterior papillary muscles. The QRS morphology during VT has an RBBB pattern with either left superior (posterior papillary muscle) or rightward inferior (anterior papillary muscle) axis.29,30 The ECG features are very similar in the LV papillary muscle and fascicular VTs; nonetheless, in contrast to fascicular VT, papillary muscle VT has a broader QRS complex (150 ± 15 milliseconds versus 127 ± 11 milliseconds). Additionally, all fascicular VTs, versus none of papillary muscle VTs, had an rsR′ pattern in lead V1. An R/S ratio not exceeding 1 in lead V6 for VTs in the LV anterolateral region also suggests papillary muscle VT.31 Furthermore, spontaneous variations in QRS morphology occur relatively frequently during VTs originating from the LV papillary muscles, a feature that can help distinguish these VTs from LV fascicular VT, the latter being a reentrant tachycardia with a consistent QRS morphology.32
Other Left Ventricular Sites of Origin
VT can originate from multiple LV sites, including the subaortic LV, the superior basal region of the left interventricular septum, papillary muscle, aortomitral continuity, mitral annulus, aortic cusps, and epicardial sites in the region of the great cardiac and anterior interventricular veins. Most of these sites of origin are associated with an LBBB pattern and inferior axis. A basal LV septal origin is suggested by LBBB morphology associated with an early precordial transition in lead V1 or V2. An origin from the aortomitral continuity is associated with RBBB morphology and broad monophasic R waves across the precordial leads. As the origin moves laterally along the mitral annulus, the R wave in lead I and in the inferior leads decreases in amplitude. LVOT free-wall VTs have an early transition and persistent dominant R wave across the precordium, with a small or absent S wave out to the apex (see Fig. 23-7). The R wave in V2 is broad and occupies a greater percentage of the QRS width than RVOT VTs. LVOT VT can occasionally have an epicardial site of origin. This form is associated with an R wave in lead V1, S wave in lead V2, precordial transition in leads V2 to V4, deep QS in lead aVL, and tall R wave in the inferior leads. Contrariwise, an R wave in lead V2 taller than in leads V1 or V3 suggests an origin in the so-called crux of the heart, near the posterior descending artery.1,33
Ventricular Tachycardias Arising from the Aortic Cusps
For LVOT VT, the absence of an S wave in leads V5 or V6 suggests a supravalvular origin, whereas the presence of such waves is consistent with an infravalvular origin (see Fig. 23-7). An R wave in lead aVL may exclude an origin in the left or right aortic cusp.16
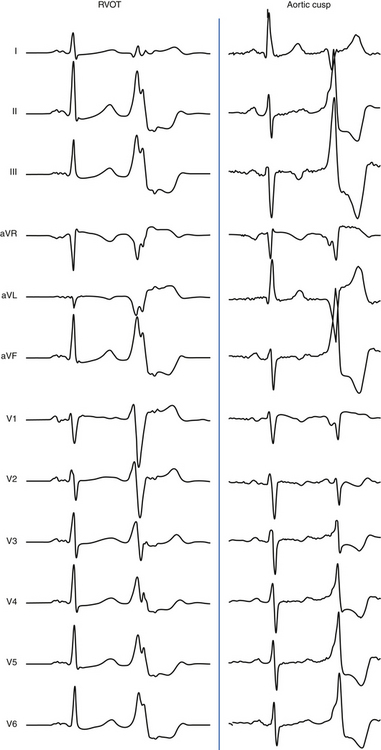
Origin from the aortic cusp is also strongly suggested by a longer duration and greater amplitude of the R wave in leads V1 and V2 (R/QRS duration >50% and R/S amplitude >30%) as compared with VT originating from the RVOT, because the aortic valve lies to the right and posterior to the RVOT. Correction of the transitional zone to cardiac rotation (i.e., comparing the R/S transitional zone in precordial leads during VT with that during NSR) can be a useful marker for differentiating an RVOT origin from an aortic cusp origin. When the transitional zone during VT occurs in one or more precordial leads earlier than during NSR, an aortic cusp origin is favored, even when the transitional zone during VT occurs later than lead V2 (Fig. 23-8).34
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree