Why Wounds Fail to Heal
Mark H. Jensen
Steven L. Moran
Surgical wound complications can be a significant problem resulting in prolonged hospital stay, reoperations, and significant morbidity for our patients. Often the surgeon can predict preoperatively which patients will have problems healing wounds or will need additional soft tissue coverage. Surgical planning should not only include the surgical approach but also an assessment of the patient’s healing risks. It is our hope that preoperative risk reduction may avoid wound healing complications.
Prevention of wound healing complications starts with an understanding of the healing process. Risk factors can be recognized and modified. Techniques and practices that are proven to prevent wound infections must be employed. This chapter will focus on understanding wound healing and identifying patients at risk for wound complications; we will also attempt to offer means to minimize risk factors for complications through surgical technique and preoperative planning.
Phases of Wound Healing
Surgically induced wounds heal in several stages. The wound passes through phases of coagulation, inflammation, matrix synthesis and deposition, angiogenesis, fibroplasia, epithelialization, contraction, and remodeling. These processes have been grouped into three main stages: inflammation, fibroplasia, and maturation. Interruption in any one of these stages can lead to wound healing complications.
The inflammatory phase of wound healing involves cellular responses to clear the wound of debris and devitalized tissue. Increased capillary permeability and leukocyte infiltration occur secondary to inflammatory mediators and vasoactive substances. Polymorphonuclear cells (PMNs) are the first cell population in the wound followed by mononuclear leukocytes which mature into wound macrophages. Inflammatory cells clean the wound of harmful bacteria and devitalized tissue. Adequate tissue oxygen tension is necessary for the release of oxygen free radicals by neutrophils. Following the initial introduction of PMNs into the wound, lymphocytes enter the wound in great number, clearing the wound of old neutrophils and secreting important cytokines and chemoattractants for fibroblasts. Fibronectin and hyaluronate deposition from fibroblasts in the first 24 to 48 hours provides scaffolding for further fibroblast migration (1,2).
The fibroblast proliferation phase starts within the initial 2 to 3 days as large populations of fibroblasts migrate to the wound. Secretion of a variety of substances necessary for wound healing and includes large quantities of glycosaminoglycans and collagen. Ground substance formed from the four main glycosaminoglycans (hyaluronic acid, chondroitin-4-sulfate, dermatan sulfate, and heparin sulfate) acts as an amorphous gel that is necessary for collagen aggregation. Collagen levels rise for approximately 3 weeks corresponding to increasing tensile strength. After 3 weeks the rate of degradation equals the rate of deposition. Angiogenesis is an important aspect of the fibroblast proliferation phase as it helps to support new cells in the healing wound.
The maturation phase starts around 3 weeks and lasts up to 2 years. It is characterized by collagen remodeling and wound strengthening. Collagen is the principal building block of connective tissue and is found in at least 13 different types. Types I to IV are the most common in the human body. Each has a distinct feature and is found in different levels in many tissues. For example, type III collagen is high in hydroxyproline and low in hydroxylysine. It is commonly found in skin, arteries, bowel wall, and healing wounds. Type I collagen is found in skin, tendon, and bone; is low in hydroxylysine content; and is the most common collagen type, accounting for more than 90% of body collagen. Early wounds are comprised of a majority of type III collagen. As the wound matures, type III collagen is replaced by type I collagen. Collagen cross-linking improves tensile strength. There is a rapid increase in strength of the wound by 6 weeks as the wound reaches 70% of the strength of normal tissue. The wound then gradually plateaus to 80% of normal strength, but never returns to preinjury levels.
Wound re-epithelialization occurs as adjacent cells migrate through a sequence of mobilization, migration, mitosis, and cellular differentiation of epithelial cells. Wound contraction starts at about 1 week. It is facilitated by the transformation of certain fibroblasts into myofibroblasts containing α×smooth muscle actin. These cells adhere to the wound margins as well as each other and effect contraction of the wound. These stages are imperative for proper wound healing as interruption of these processes results in chronic wound complications (1,3).
Risk Factors
The identification of patients at risk for aberrant wound healing allows the surgeon to make appropriate plans for skin closure technique, flap utilization, and postoperative wound management. This will ideally result in modification of risk factors prior to surgery. In cases of chronic diseases or nonmodifiable risk factors, patients must be informed of increased wound healing risks. The following discussion focuses on commonly encountered risk factors with recommendations to ameliorate their effects.
Diabetes
Patients with diabetes are both more likely to undergo surgery and develop perioperative complications than nondiabetic patients. This leads to longer hospital stay with higher health care costs and increased perioperative mortality. Diabetic patients have increased rates of hypertension, cardiac disease, and renal failure. These factors lead to much higher wound healing complications (3). Diabetes inhibits wound healing through many mechanisms. It is a disease affecting small vessels which are critical in supplying nutrients to the healing wound. Elevated glucose levels also affect a myriad of inflammatory systems. Neutrophil adherence, chemotaxis, phagocytosis, and intracellular bactericidal activity are all impaired. Pseuodhypoxia develops as a result of altered redox reactions and vascular permeability secondary to hyperglycemia. Furthermore, glucose is a proinflammatory mediator stimulating cytokine production and inhibiting endothelial nitric oxide levels (4). This translates clinically into higher infectious complications. Tight control of glucose in the perioperative period mitigates the postoperative complications seen in the diabetic patient. This has been found in both the intensive care setting as well as routine operative cases (3,5).
Management of patients with diabetes starts in the pre-operative period. There is good evidence to suggest that a preoperative hemoglobin A1C of less than 7% drastically decreases postoperative infectious complications (4). Physicians should aggressively improve glucose management using diet, oral hypoglycemic agents, and insulin as needed. Perioperative management should include sliding scale insulin or continuous insulin infusion to maintain glucose levels below 150 mg per deciliter (3). Evidence suggests that improved outcomes are possible with tighter control of glucose levels between 80 and 110 mg per deciliter in critically ill patients (5). After dismissal from the hospital, the patient should continue their preoperative diabetic regiment.
Obesity
Obese patients have a higher rate of wound infections, dehiscence, hematomas, seromas, and pressure ulcers (6×8). This is felt to be due to multiple factors including difficulty with the operation, altered immune response, increased tension with closure, increased dead space, decreased microperfusion, and decreased mobility post operatively (9,10). Patients should be encouraged to lose weight prior to elective operations. Increased levels of physical activity preoperatively translates to an improved postoperative rehabilitation process. Successful weight loss is difficult to achieve in most patients. This has prompted some surgeons to recommend gastric bypass surgery prior to certain operations such as joint replacement. This has been associated with improved outcomes in hip replacement patients with morbid obesity (11).
Smoking
Numerous studies have consistently found that smokers have significantly higher rates of wound healing complications than nonsmokers. This is related to several causes including the vasoactive effect of cigarette smoke through the sympathetic alpha receptors, increased levels of carboxyhemoglobin with a reduction in oxygen-carrying capacity, increased platelet activation leading to microangiopathic thrombosis, increased levels of fibrinogen with decreased fibrinolytic activity, endothelial injury, and increased hemoglobin levels leading to increased blood viscosity. Regardless of the mechanism the effect is significant. Complication rates of 2.5% to 6% in nonsmokers versus 7.5% to 49% in smokers are reported in the literature.
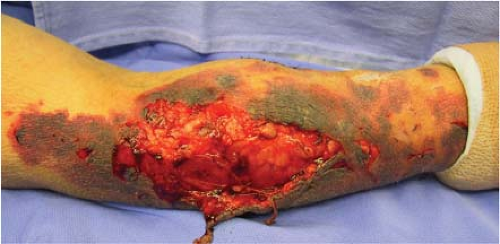
The increased risk of wound complications in smokers is most pronounced in cases where a large area of tissue is undermined. This is most likely related to failure of the dermal and subdermal plexus supplying the resultant skin flap (Fig. 1-1). Poor outcome in smokers prompts many clinicians to postpone elective procedures until the patient has quit smoking for at least 3 to 4 weeks, particularly if the procedure involves large areas of undermining (12,13). Aggressive use of smoking cessation
programs should be implemented. Still, other surgeons feel that this would restrict too many patients from receiving necessary surgery (14,15). We prefer to delay surgery for 4 weeks prior to elective surgical procedures where soft tissue coverage may be an issue and advocate early referral to a nicotine dependence unit. If smoking cessation is not possible, the patient is informed of the likelihood of additional flap coverage for wound closure and a higher likelihood of postoperative wound complications.
programs should be implemented. Still, other surgeons feel that this would restrict too many patients from receiving necessary surgery (14,15). We prefer to delay surgery for 4 weeks prior to elective surgical procedures where soft tissue coverage may be an issue and advocate early referral to a nicotine dependence unit. If smoking cessation is not possible, the patient is informed of the likelihood of additional flap coverage for wound closure and a higher likelihood of postoperative wound complications.
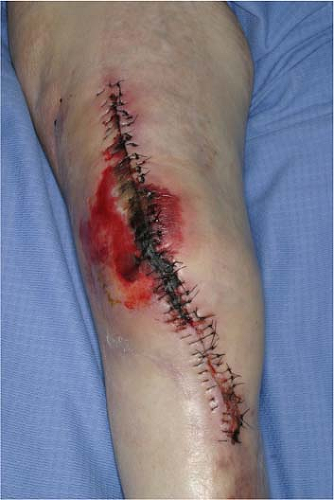 FIGURE 1-1 Following total knee arthroplasty this 76-year-old diabetic smoker developed wound complications necessitating wound debridement and flap coverage. |
Immunosuppressive Medications
Patients with intrinsic or acquired immunodeficiencies are at increased risks for wound healing complications. Patients with MHC class-II deficiency have impaired wound healing because of altered T cell immune function (16). Patient populations at risk for wound healing problems due to altered immune function include patients with hereditary, infectious, and iatrogenic immune deficiencies.
Corticosteroids inhibit wound healing by their anti-inflammatory effect. Decreased numbers of inflammatory cells are noted at the wound site; delays in collagen synthesis, fibroblast proliferation, angiogenesis, wound contracture rates, and epithelial migration are also observed (Fig. 1-2). Vitamin A has been shown to counteract the effects of steroids on wound healing in all areas except wound contraction and infection (17). The exact mechanism is not known, but may be related to the TGF-beta, IGF-I, and hydroxyproline content in the tissue (18). Factors that can help improve wound healing in immunocompromised states include prevention of malnutrition, hypoxia, endocrine disorders, anemia, and other metabolic disorders. Dead tissue, foreign bodies, tissue ischemia, and hematoma should be minimized.
Radiation and Chemotherapy
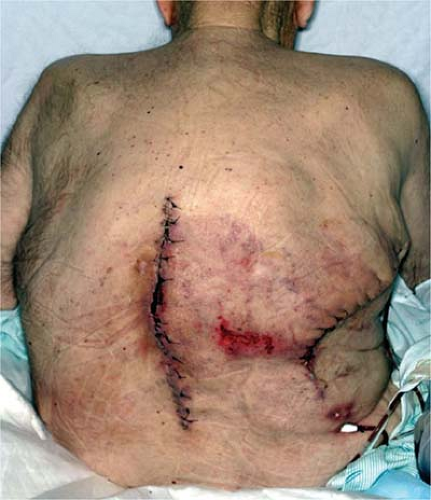
Radiation and chemotherapy are known to cause delays in wound healing. Operations in irradiated fields are particularly problematic due to dense fibrosis and decreased perfusion caused by small vessel injury (Fig. 1-3). Irradiated fields are more susceptible to infection and delayed healing. Postoperative radiation initiated after the initial 3 to 4 weeks of primary wound healing does not seem to have as marked an effect on wound healing, but can lead to contracture, wound break down, and flap necrosis (21). Neoadjuvant chemotherapy can alter wound healing, especially in cases where chemotherapy has led to neutropenia. Surgery would be ideally delayed until full recovery of platelets and leukocytes. Healing seems to proceed normally in patients who receive their chemotherapy 3 to 4 weeks after surgery, as the wound has been allowed to proceed through the first stages of healing (8,22). Certain chemotherapeutic agents, however, can have a negative effect on wound healing far greater than 4 weeks; for example, Avastin (Bevacizumab), which inhibits vascular endothelial growth factor, has an extremely long half life ranging from day 11 to 50 and has been shown to inhibit wound healing when given in the neoadjuvant setting (23). Surgeons should have a good understanding of specific complications associated with individual chemotherapeutic regiments.
Malnutrition
It has been known for many years that malnutrition has deleterious effects on wound healing. Loss of nutrients alters host immunity through decreased T-cell function, phagocytosis, complement, and antibody levels. Certain patient groups are at particular risk of malnutrition. Severe catabolic states can be induced after multi-system trauma, sepsis, and burns. Significant increases in metabolic rate
can also follow uncomplicated abdominal surgery (increase by 10%), uncomplicated injuries such as femoral fracture (20%), peritonitis (40%), and fever (10% for every 1°C above normothermia). This is particularly alarming considering the high portion of trauma patients who are unable to eat for extended periods leading up to and following surgery (24,25).
can also follow uncomplicated abdominal surgery (increase by 10%), uncomplicated injuries such as femoral fracture (20%), peritonitis (40%), and fever (10% for every 1°C above normothermia). This is particularly alarming considering the high portion of trauma patients who are unable to eat for extended periods leading up to and following surgery (24,25).
 Get Clinical Tree app for offline access 
|