Management of Simple Wounds: Local Flaps, Z-Plasty, and Skin Grafts
John B. Hijjawi
Allen T. Bishop
Skin grafting and local flap coverage have remained a common means of covering traumatic wounds. Skin grafts, local flaps, and random flaps are not as simple as direct wound closure nor are they are as “elegant” as procedures that are found higher on the reconstructive ladder such as pedicled flaps or free tissue transfer (Fig. 3-1). However, the procedures described in this chapter remain straightforward, reliable, and time-tested and should be an essential component of any surgeon’s reconstructive armamentarium.
Skin Grafts
Skin grafts are classified by source and thickness. By far the most common, durable, and successful skin grafts are autografts harvested from the patient’s own skin. There are no immunologic issues, since the tissue comes from the patient’s own body. Concerns over disease transmission are eliminated and expense is minimal. The only disadvantage is the creation and care of a donor site.
Skin grafts are also available as cadaveric allografts, xenografts (typically porcine skin), and most recently, cultured epithelial grafts. These materials are lifesaving sources of temporary wound coverage in the context of massive burns. Allografts and xenografts do have the disadvantage of immunogenicity and thus impermanence since they are bound to be rejected. However, if the quality of a wound bed is questionable, preserved porcine xenografts may be an excellent option for temporary wound coverage.
Currently available cultured epithelial grafts are expensive, require time to culture, and are not as durable as autografts. Additionally, studies have shown that they are significantly more susceptible to infection than standard autografts.
Skin grafts are also classified based on thickness. The skin is composed of an outer epidermis and a deeper dermis, which is further subdivided into the reticular and papillary dermis (Fig. 3-2). All skin grafts consist of the entire epidermis and a variable amount of dermis. Split-thickness skin grafts contain only a portion of the dermis, whereas full-thickness skin grafts contain the entire dermis and dermal appendages. As a result, full-thickness skin grafts continue to support hair growth following transfer. This needs to be carefully considered when selecting donor sites in situations when a full-thickness skin graft is to be transferred to a conspicuous, previously hairless area.
Since full-thickness skin grafts include all dermal appendages, skin at the donor site will not spontaneously regenerate following harvest; therefore, these donor sites need to be closed primarily or with a split-thickness skin graft. Split-thickness donor sites retain the ability to generate epithelium and will be largely healed within several weeks if cared for properly.
Split-thickness and full-thickness skin grafts have quite different contractile characteristics on harvesting (primary contraction) and after they heal (secondary contraction). Primary contraction is
the initial contraction of a graft when it is harvested. Due to the greater proportion of elastic fibers in a full-thickness skin graft (the dermis is the location of all elastic fibers in the skin), it will undergo more primary contraction than a split-thickness skin graft when harvested. Similarly, a thicker split-thickness skin graft (e.g., 0.018 in) will undergo more primary contraction than a thin split-thickness skin graft. Most skin grafts can be stretched under minimal tension at inset to overcome this contraction, restoring their original size.
the initial contraction of a graft when it is harvested. Due to the greater proportion of elastic fibers in a full-thickness skin graft (the dermis is the location of all elastic fibers in the skin), it will undergo more primary contraction than a split-thickness skin graft when harvested. Similarly, a thicker split-thickness skin graft (e.g., 0.018 in) will undergo more primary contraction than a thin split-thickness skin graft. Most skin grafts can be stretched under minimal tension at inset to overcome this contraction, restoring their original size.
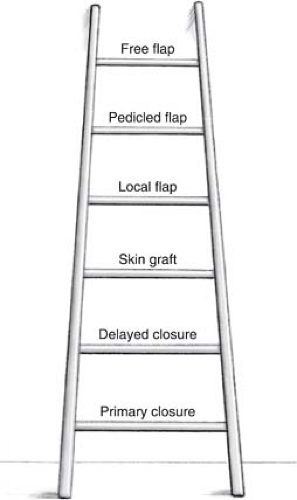 FIGURE 3-1 Reconstructive ladder. Historically, surgeons have closed wounds with the simple procedure first, moving up the rungs of the reconstructive ladder as wounds become larger and more complex. |
Conversely, split-thickness skin grafts undergo more secondary contraction than full-thickness grafts. This can be exploited to provide gradual contraction of a wound over the course of several months. An example is a fasciotomy wound that is under too much tension to close primarily within the days following compartment release. A very thin split-thickness skin graft applied to the wound will undergo significantly more secondary contraction than would a full-thickness skin graft, resulting in contraction of the wound itself. After several months, this may result in a wound that is small enough to allow serial excision of the skin graft and primary closure under minimal tension. In comparison,
full-thickness skin grafts can be relied on to undergo virtually no secondary contraction in situations where this is not desirable, such as across a joint surface or in a web space.
full-thickness skin grafts can be relied on to undergo virtually no secondary contraction in situations where this is not desirable, such as across a joint surface or in a web space.
Initial adhesion of a skin graft is the result of fibrin present between the graft and the recipient bed. Initial survival of a skin graft relies on plasmatic imbibition, which is the process of nutrient diffusion from the recipient site into the skin graft. Later, vascular channels within the graft line up with vascular channels in the recipient bed through the process of inosculation. Finally, long-term graft survival relies on neovascularization, or the process of new blood vessel growth into the skin graft from the recipient bed. Because skin grafts, both split thickness and full thickness, are completely reliant on the recipient bed for survival, the wound bed must be well-vascularized and free of infection to support skin graft take. Split-thickness skin grafts have lower metabolic demands than do full-thickness grafts, and so they don’t require recipient beds with as rich a blood supply. Along the same lines, full-thickness grafts take longer, from 7 to 10 days, to heal. Split-thickness grafts are generally considered healed by 5 days and should be left immobilized and dressed at least that long postoperatively. The time of healing in specific situations depends most on the quality of the recipient wound’s blood supply.
Indications/Contraindications
Indications
Skin grafting may be indicated for any defect that cannot be closed primarily and that has a wound bed that can support skin graft take (Table 3-1). Skin grafts survive for the first several days through a process called imbibition. During this stage of skin graft healing, the graft obtains nutrients from the underlying wound bed through a process of diffusion. Wound beds devoid of blood flow, or with little vascularized tissue, will make for poor recipient sites. Exposed structures that will accept a graft include subcutaneous tissue, paratenon, and muscle. Other tissues, such as exposed bone, joint, tendon, and nerve, may be covered temporarily by graft used as a biologic dressing but will not support a graft for permanent coverage. Beds containing tissues of questionable viability, chronic granulation tissue, or frank infection can accept a skin graft but require thorough debridement prior to graft placement. Wounds that contain fewer than 105 bacteria per gram of tissue or that allow xenograft adherence within 24 hours allow successful skin grafting.
Contraindications
Skin grafts are contraindicated in areas that are exposed to repetitive trauma or that lie over osseous prominences. Bone, cartilage, and tendons denuded of periosteum, perichondrium, or paratenon cannot be covered with skin grafts as there is inadequate vascular supply to support healing of the skin graft. Controversy exists over whether skin grafts should be placed over bone, cartilage, or tendons with healthy periosteum, perichondrium, or paratenon. It is certainly possible to get skin-graft healing over such structures. However, skin grafts in these situations are rarely optimal for long-term durable coverage. In addition, skin grafting should be avoided in areas that may require secondary surgery for bone or nerve grafting, as adherence to underlying muscle, nerve, and tendon may complicate secondary surgery. All split thickness grafts will undergo some component of contracture over time; thus, if these grafts are placed over large areas of the antecubital fossa, popliteal fossa, or olecranon, there is a risk of limitation in joint motion.
Preoperative Planning
The wound must be debrided and clean prior to attempts at skin grafting. Infection is one of the leading causes of skin graft failure. Since skin grafts are completely dependent on the wound bed they are transplanted to for nutrition, they possess no intrinsic ability to resolve infection. Quantitative wound cultures have been used for many years in some centers to determine the adequacy of a wound’s microenvironment for closure. A quantitative culture revealing less than 105bacteria per gram of tissue has been traditionally regarded as an acceptable level of colonization below which a
wound can be closed by skin graft or local flap. However, such cultures are highly dependent on the experience of the technician performing them. A careful clinical evaluation and serial sharp debridement of clinically infected or contaminated wounds are advised.
wound can be closed by skin graft or local flap. However, such cultures are highly dependent on the experience of the technician performing them. A careful clinical evaluation and serial sharp debridement of clinically infected or contaminated wounds are advised.
Table 3-1. Wound Analysis | |
|---|---|
|
Donor site selection must also be decided before surgery. Considerations when choosing a donor site for split thickness skin grafts include cosmesis, the thickness of skin in the donor site region, and ease of care of the donor site after graft harvest. The most common split thickness skin donor sites include the buttocks, lateral and anterior thighs, and lower abdomen. These sites are relatively easy to conceal, have thick skin resulting in less pigmentation once healed, and are readily accessible in even a bed-bound patient, easing postoperative care.
Full thickness skin grafts are most commonly harvested from the groin, antecubital fossa, volar wrist crease, medial arm, postauricular sulcus, or lower abdomen. These donor sites all exist in areas where closure can be performed within pre-existing skin creases, thus resulting in relatively inconspicuous donor sites. The hypothenar skin or plantar instep offers the unique quality of glabrous skin if needed for graft material. Always keep in mind that any amputated “spare parts” can provide a good source of viable skin graft with no added morbidity to the patient.
Surgery
Patient Positioning
The patient’s position will depend on the location of the graft to be harvested. Most frequently we harvest split thickness grafts from the upper thigh area, where they may easily be concealed under clothing A supine position with a roll underneath one hip is ideal. The majority of full-thickness grafts are harvested from the hairless skin of the groin crease, though the inner upper arm may be used as well. The patient is positioned supine for such harvests. For glaborous skin grafts, the instep of the foot may be positioned so as to allow ease of harvest. For wounds on the posterior aspect of the lower extremity or trunk, a lateral decubitus position readily exposes both the wound and a lateral thigh donor site. With careful planning it is almost never necessary to reposition a patient after harvesting the skin graft.
Split Thickness Grafts
Power dermatomes are the most common method of harvesting split thickness skin grafts, although for very small split thickness grafts hand-driven Weck blades may be more convenient.
Measure the recipient wound and choose a dermatome guard based on that measurement (Fig. 3-3).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree









