The prevalence of peripheral arterial disease is high and will continue to grow with our aging population. It is often under diagnosed and under treated due to a general lack of awareness on the part of the patient and the practitioner. The evidence-base is growing for the optimal medical management of the patient with peripheral arterial disease; in parallel, endovascular revascularization options continue to improve. Exercise training for claudication rehabilitation plays a critical role. Comprehensive care of the peripheral arterial disease patient focuses on the ultimate goals of improving quality of life and reducing cardiovascular morbidity and mortality.
Update on peripheral arterial disease
Peripheral arterial disease (PAD) is an atherosclerotic syndrome in which the lumen of the arteries in the lower extremities becomes progressively obstructed by plaque. Although technically PAD can also refer to disease in other vascular beds, exclusive of the coronary vessels, in this article the authors refer to PAD as an arterial occlusive disease affecting the lower extremity arteries.
Epidemiology and risk factors
Recent epidemiologic projections indicate a prevalence of PAD of 11% to 16% in the population aged 55 years or older, affecting an estimated 27 million persons in Europe and North America alone. Moreover, the prevalence of PAD may be as high as 20% to 30% in specific high-risk populations. The reported prevalence of PAD depends on the population selected for testing and the methods used for diagnosis. At the start, intermittent claudication questionnaires were used to estimate the prevalence of PAD in a specific population. Later, a noninvasive test known as the ankle-brachial index (ABI) was developed for a more objective assessment of PAD. The ABI is calculated by dividing the ankle systolic pressure by the higher brachial systolic pressure, with an ABI less than 0.9 considered up to 95% sensitive and 99% specific for angiographically confirmed lower extremity arterial disease and a reported positive predictive value of 90%, a negative predictive value of 99%, and an overall accuracy of 98%. One study conducted among 613 individuals living in Southern California used several different techniques to assess the presence of PAD. The study reported that the use of a claudication questionnaire alone significantly underestimated the prevalence of PAD, whereas the use of the ABI combined with pulse wave velocity measurements increased the detection of PAD 2 to 7 times more than claudication symptoms alone. In this study, the prevalence of PAD based on ABI less than 0.9 was 18.8% among those aged 70 years or older, in comparison to a prevalence of 2.5% in those aged 60 years or younger.
Other epidemiologic studies have also described an increase in the prevalence of PAD with increasing age, 1 of the most important risk factors for the development of PAD. For example, the National Health and Nutrition Examination Survey (NHANES), a cross-sectional US survey of 2174 individuals, reported a prevalence of PAD (defined by ABI <0.9) of 14.5% in those aged 70 years or older, compared with a prevalence of 4.3% in the overall population aged 40 years or older. Male gender was previously considered an important risk factor for PAD, but subsequent studies have shown that while men are slightly more affected than women in the younger age groups, the distribution of PAD in the older age groups seems equal between genders.
In epidemiologic studies, race-ethnicity has also emerged as a determining factor for the presence of PAD. NHANES reported that the prevalence of PAD in non-Hispanic blacks was as high as 7.8%, compared with 4.4% in whites. The Multi-Ethnic Study of Atherosclerosis (MESA) also reported a higher prevalence of PAD in blacks compared with whites and Hispanics. Although NHANES and MESA showed a lower prevalence of PAD in Hispanics compared with non-Hispanic whites, Morrissey and colleagues reported that Hispanics present with more advanced stages of disease, demonstrating higher rates of limb-threatening ischemia, failed lower extremity revascularization, and amputations than non-Hispanic whites.
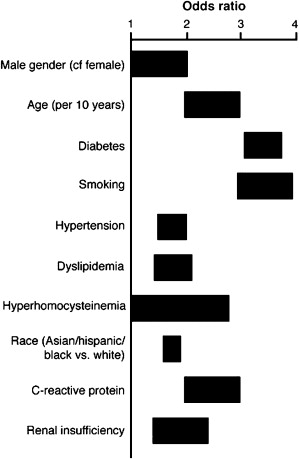
As illustrated in Fig. 1 , additional risk factors that increase the likelihood of PAD are similar to traditional risk factors for atherosclerosis, including smoking, diabetes mellitus, hypertension, dyslipidemia, chronic kidney disease, hyperhomocysteinemia, and elevated C-reactive protein. Of those, the 2 most strongly associated with PAD are smoking and diabetes. Up to 80% of PAD patients report being current or former smokers. Smoking increases the risk of PAD by 2- to 6-fold, and is twice more likely to cause PAD than coronary artery disease (CAD). Diabetes mellitus, present in up to 20% of patients with PAD, increases the risk of PAD by up to 4-fold; in addition, PAD patients with diabetes are at increased risk for developing lower extremity complications such as major limb amputations.
Epidemiology and risk factors
Recent epidemiologic projections indicate a prevalence of PAD of 11% to 16% in the population aged 55 years or older, affecting an estimated 27 million persons in Europe and North America alone. Moreover, the prevalence of PAD may be as high as 20% to 30% in specific high-risk populations. The reported prevalence of PAD depends on the population selected for testing and the methods used for diagnosis. At the start, intermittent claudication questionnaires were used to estimate the prevalence of PAD in a specific population. Later, a noninvasive test known as the ankle-brachial index (ABI) was developed for a more objective assessment of PAD. The ABI is calculated by dividing the ankle systolic pressure by the higher brachial systolic pressure, with an ABI less than 0.9 considered up to 95% sensitive and 99% specific for angiographically confirmed lower extremity arterial disease and a reported positive predictive value of 90%, a negative predictive value of 99%, and an overall accuracy of 98%. One study conducted among 613 individuals living in Southern California used several different techniques to assess the presence of PAD. The study reported that the use of a claudication questionnaire alone significantly underestimated the prevalence of PAD, whereas the use of the ABI combined with pulse wave velocity measurements increased the detection of PAD 2 to 7 times more than claudication symptoms alone. In this study, the prevalence of PAD based on ABI less than 0.9 was 18.8% among those aged 70 years or older, in comparison to a prevalence of 2.5% in those aged 60 years or younger.
Other epidemiologic studies have also described an increase in the prevalence of PAD with increasing age, 1 of the most important risk factors for the development of PAD. For example, the National Health and Nutrition Examination Survey (NHANES), a cross-sectional US survey of 2174 individuals, reported a prevalence of PAD (defined by ABI <0.9) of 14.5% in those aged 70 years or older, compared with a prevalence of 4.3% in the overall population aged 40 years or older. Male gender was previously considered an important risk factor for PAD, but subsequent studies have shown that while men are slightly more affected than women in the younger age groups, the distribution of PAD in the older age groups seems equal between genders.
In epidemiologic studies, race-ethnicity has also emerged as a determining factor for the presence of PAD. NHANES reported that the prevalence of PAD in non-Hispanic blacks was as high as 7.8%, compared with 4.4% in whites. The Multi-Ethnic Study of Atherosclerosis (MESA) also reported a higher prevalence of PAD in blacks compared with whites and Hispanics. Although NHANES and MESA showed a lower prevalence of PAD in Hispanics compared with non-Hispanic whites, Morrissey and colleagues reported that Hispanics present with more advanced stages of disease, demonstrating higher rates of limb-threatening ischemia, failed lower extremity revascularization, and amputations than non-Hispanic whites.
As illustrated in Fig. 1 , additional risk factors that increase the likelihood of PAD are similar to traditional risk factors for atherosclerosis, including smoking, diabetes mellitus, hypertension, dyslipidemia, chronic kidney disease, hyperhomocysteinemia, and elevated C-reactive protein. Of those, the 2 most strongly associated with PAD are smoking and diabetes. Up to 80% of PAD patients report being current or former smokers. Smoking increases the risk of PAD by 2- to 6-fold, and is twice more likely to cause PAD than coronary artery disease (CAD). Diabetes mellitus, present in up to 20% of patients with PAD, increases the risk of PAD by up to 4-fold; in addition, PAD patients with diabetes are at increased risk for developing lower extremity complications such as major limb amputations.
Clinical manifestations of PAD
Intermittent Claudication and Atypical Leg Symptoms
Intermittent claudication, defined as reproducible pain in the lower limbs during exercise relieved by rest, is the most common manifestation of symptomatic PAD. Claudication most commonly refers to pain in the lower extremity muscle groups, though other symptoms such as fatigue, weakness, or other discomfort may also occur. Although patients with symptomatic PAD often have sufficient blood flow at rest to avoid limb discomfort, blood flow during exercise is insufficient to meet increased metabolic demands and muscle pain develops. Pain in specific muscle groups is frequently associated with the anatomic site of arterial occlusive disease. For example, pain in the muscles of the buttocks or thighs may represent aortoiliac disease, whereas pain in the calf is most commonly associated with stenosis of the femoral or popliteal arteries.
Several questionnaires have been developed for use in epidemiologic assessments of intermittent claudication. The earliest of these is the Rose Claudication Questionnaire, which has been demonstrated to have high specificity but low sensitivity. Although claudication is considered the main symptom of PAD, it is important to remember that the prevalence of intermittent claudication is low among patients diagnosed with PAD and that several studies have shown that physicians who rely on a history of claudication alone to detect PAD will miss most cases. The Rotterdam Study, which examined 7715 elderly subjects in a population-based study, reported a prevalence of PAD (defined as ABI <0.9) of 19.1%. However, only 1.6% of individuals with PAD reported symptoms of claudication based on the Rose Questionnaire. Newer and more sensitive questionnaires have been developed to determine the presence of lower extremity symptoms, allowing for the assessment of atypical leg pain. For example, the San Diego Questionnaire was developed to categorize patients as having intermittent claudication, atypical leg pain, or no leg pain. This questionnaire was used in the PAD Awareness, Risk and Treatment: New Resources for Survival (PARTNERS) program in which only 8.7% of patients with PAD had a positive Rose Questionnaire. More than half of the patients with PAD exhibited atypical leg symptoms by the San Diego Questionnaire.
Although patients with PAD may have claudication or atypical leg pain, these symptoms are not often brought to the attention of their physicians for a variety of reasons. One study reported that up to one third of patients do not alert a physician to their leg symptoms as they attribute them to musculoskeletal pain, arthritis, or aging. In that same study, patients with objective PAD also demonstrated 1 or more comorbidities such as neuropathy, arthritis, and spinal stenosis. These comorbidities can mask or alter the symptoms of claudication and, as a consequence, physicians may be less likely to consider the diagnosis of PAD in these patients.
Asymptomatic Functional Impairment
Although many patients with PAD may not exhibit symptoms of claudication, evidence suggests that some degree of functional impairment is usually present and that whether or not symptoms develop may depend on the patient’s degree of physical activity. The Women’s Health and Aging Study examined the lower extremity functioning of 933 women, 328 of whom had PAD as defined by ABI less than 0.9. In the cohort of patients with PAD, 63% reported no leg symptoms with exertion and were therefore classified as asymptomatic; however, after objective testing, these individuals were found to have worse lower extremity functioning than an age-matched cohort. Women who were classified as having asymptomatic PAD were actually found to have slower walking velocities and poorer standing balance scores, and walked fewer blocks per week than women without PAD, after adjustment for other comorbidities. In another cross-sectional study of 460 subjects with PAD and 130 subjects without PAD, McDermott and colleagues reported decreased functional status in subjects with PAD who reported no lower extremity symptoms with exertion. In fact, most subjects with asymptomatic PAD developed symptoms during a 6-minute walk test, suggesting that they were restricting their physical activity in daily life to avoid exertional leg pain. Although a significant number of patients with PAD may be classified as asymptomatic, recent research points to the fact that impaired lower extremity functioning is almost always present in these individuals.
Critical Limb Ischemia
Critical limb ischemia (CLI) is defined by pain in the lower extremities that occurs with rest or evidence of tissue loss in the setting of severely compromised arterial flow. In individuals with CLI, occlusive disease progresses to the point that blood flow at rest becomes insufficient for tissue viability, and chronic rest pain, ulcers, or gangrene can develop. The natural history of CLI is not well defined as most of these patients now receive early invasive treatment. However, if untreated, the expected outcome would be major limb amputation within 6 months. Patients with CLI usually present with foot pain at rest that may worsen in the supine position and may improve when the limb is placed in a dependent position. Although fewer than 5% to 10% of patients with PAD have CLI, patients with diabetes are particularly prone to developing this syndrome, which is associated with a substantial risk of limb loss. Acute limb ischemia (ALI) occurs due to a sudden deterioration in limb perfusion and may be a form of CLI but is more commonly related to an acute event such as embolism or local thrombosis. Patients with ALI often present with the 5 “Ps” of pain, pallor, paresthesias, pulselessness, and poikilothermia and commonly report no prior history of claudication. ALI, which is associated with a high mortality and significant risk of limb loss, should be treated emergently, usually with a combination of anticoagulation therapy, thrombolysis, and open surgical repair.
Natural history of PAD
Cardiovascular Outcomes
Given the coexistence of coronary and cerebrovascular disease in patients with PAD, as well as a well-described association between ABI and cardiovascular morbidity and mortality, patients with PAD are considered at significantly increased risk for myocardial infarction, stroke, and vascular death over a 5-year period in comparison to age-matched cohorts. The overall 5-year mortality for PAD patients is approximately 15% to 30%, with more than 75% attributable to cardiovascular causes.
PAD as a marker for disease in other vascular beds
Prior studies have demonstrated that patients with symptomatic atherosclerotic disease in 1 vascular bed are at risk for polyvascular disease. For example, 26% of patients analyzed as part of the Clopidogrel versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) trial had symptomatic disease in at least 2 vascular beds. The Swiss Atherothrombosis Survey revealed that 52% of symptomatic PAD patients reported a concurrent history of stroke/transient ischemic attack (TIA) or CAD and that 23% of patients with a history of stroke/TIA or CAD were found to have previously undiagnosed, asymptomatic PAD. The recent Reduction of Atherothrombosis for Continued Health (REACH) international registry which includes 63,000 patients from 43 countries has demonstrated that 63% of approximately 7000 patients with PAD have concomitant symptomatic cerebrovascular or coronary disease.
The ABI and vascular events
The Framingham Heart Study initially described the association between intermittent claudication and both coronary heart disease and stroke and reported a 2-fold age-adjusted increased risk of death in men and women with claudication. The presence of PAD as defined by an ABI less than 0.9 has been associated with a 6-fold increased risk of cardiovascular death at 10 years and a 2- to 3-fold increased risk of ischemic stroke when compared with the general population. Although an ABI between 0.9 and 1.3 has been traditionally considered normal, the MESA has reported excess coronary and carotid atherosclerosis in subjects with low-normal ABIs (0.9–1.1) and high ABIs (>1.3), suggesting that even subjects with borderline or low-normal ABIs may be at increased risk for cardiovascular events. Medial calcification, or incompressible arteries, common in the elderly and in patients with long-standing diabetes or chronic kidney disease, may also be associated with higher cardiovascular risk. For example, the Strong Heart Study reported an increased risk of all-cause and cardiovascular mortality for subjects with a low ABI (<0.9), borderline ABI (0.9–1.0), and high ABI (>1.4).
Limb Outcomes
For PAD patients presenting with CLI, the prognosis of the limb is poor in the absence of timely revascularization. However, in patients initially presenting with intermittent claudication or atypical leg pain, limb symptoms typically stabilize over time, with only 10% to 20% of patients experiencing worsening claudication and fewer than 2% progressing to CLI. For patients with PAD who are initially asymptomatic, a degree of functional decline over time has been described. One study revealed that PAD patients who were asymptomatic at baseline had significantly greater decline in walking performance at 2 years than those without PAD.
Noninvasive diagnosis of PAD
History and Physical Examination
The diagnosis of PAD should begin with a thorough history. PAD should be considered in patients presenting with symptoms suggestive of claudication or in patients with multiple risk factors for PAD and atypical leg pain. The differential diagnosis of exertional leg pain also includes spinal stenosis, osteoarthritis, peripheral neuropathy, and venous claudication.
The physical examination should be conducted with the patient in a gown with shoes and socks off, with a focus on skin changes, pulses, and the presence or absence of ulcers. Skin findings common in PAD include abnormal color (red or dusky purple) or pallor, hair loss on legs and toes, cool skin, and atrophic nail changes. In the setting of severe arterial insufficiency, ulcers may be present either on the toes or on the lateral malleolus. Arterial ulcers are usually painful (unless the patient has a concomitant peripheral neuropathy) and may be associated with a surrounding cellulitis or gangrene. Assessment of the femoral, popliteal, posterior tibial, and dorsalis pedis pulses should also be part of the physical examination. The femoral pulse can be palpated inferior to the inguinal ligament. The absence of a femoral pulse may indicate a stenosis or occlusion of the distal aorta or iliac artery. The popliteal pulse is felt in the popliteal fossa behind the knee. A prominent popliteal pulse may be suggestive of a popliteal artery aneurysm, while a diminished or absent pulse may indicate more proximal stenosis. The posterior tibial artery is found posterior to the medial malleolus and the dorsalis pedis artery on the dorsum of the foot.
It is important to remember that a pulse examination alone is not sufficient to establish the diagnosis of PAD. Criqui and colleagues reported that an abnormal dorsalis pedis artery pulse was 50% sensitive and 73% specific for the detection of PAD, whereas an abnormal posterior tibial artery pulse demonstrated a sensitivity of 71% and a specificity of 91%. A handheld Doppler device, along with acoustic gel, can be used to assess the arterial signal. A normal arterial signal contains 3 components and is described as triphasic. In the presence of disease, the arterial signal is altered and may only have 2 components (biphasic) or 1 component (monophasic). In cases of severe disease or occlusion, the arterial signal may be absent. Other physical findings that may be suggestive of PAD include the presence of a bruit by auscultation of the iliac, femoral, or popliteal arteries. One study which reviewed the accuracy of the clinical examination for PAD reported that the presence of a femoral bruit significantly increases the likelihood of PAD; however, its absence does not affect the probability that PAD is present.
ABI
The ABI is considered the most effective PAD screening test. The ABI is measured after the patient has been lying supine for 5 to 10 minutes. The systolic pressure is measured at the bilateral brachial, dorsalis pedis, and posterior tibial arteries using appropriately sized (10–12 cm) sphygmomanometer cuffs and a handheld 5- or 10-MHz Doppler probe with acoustic gel ( Fig. 2 ). Typically, the ABI for each leg is calculated by dividing the higher of the 2 ankle pressures in that leg by the higher of the 2 brachial pressures. The lower of the left and right ABI values is reported as the ABI. An ABI less than 0.9 is diagnostic of PAD and also gives information regarding the severity of disease; specifically, an ABI of 0.7 to 0.89 is considered mild, an ABI of 0.4 to 0.69 is moderate, and an ABI less than 0.4 is severe. The range for a normal ABI is 0.9 to 1.3, though the range from 0.9 to 1.0 is considered borderline or indeterminate ( Fig. 3 ).

The ABI has some significant limitations. In some patients, such as the elderly and those with diabetes or chronic kidney disease, calcified or noncompressible vessels can lead to falsely elevated ankle pressures, which may result in an artificially “normal” ABI or a high ABI (>1.3). In certain cases, medial calcification may cause difficulty in abolishing the systolic pressure signal despite inflating the cuff to pressures more than 250 mmHg. Another major limitation of the ABI is that it may seem normal at rest in patients with aortoiliac disease, usually when collaterals are present. The ABI only becomes abnormal with exercise when areas of stenosis or occlusion become hemodynamically significant. Thus, it is important not to exclude the diagnosis of PAD when the resting ABI is normal, particularly if symptoms suggestive of claudication are present. In these instances, the patient may be asked to exercise (usually on a treadmill at 1.5–2 mph at a 12% grade) until the claudication symptoms are reproduced, for a maximum of 5 minutes. The ankle pressure is measured again following exercise, with a 15% to 20% subsequent drop in the ABI considered diagnostic of PAD.
Noninvasive Physiologic Testing
Noninvasive physiologic testing, when added to the ABI, can provide additional key information, including determination of the location and severity of PAD. The components of physiologic testing include segmental limb pressures and pulse volume recordings (PVRs). Continuous wave Doppler tracings are also an alternative to PVRs. Segmental limb systolic pressures are measured using sphygmomanometer cuffs at the thigh, calf, and ankle levels. PVRs are obtained using plethysmography, a technique whereby air is inflated into each cuff up to a pressure of 65 mmHg. The transient volume change beneath the cuff is then translated into a pulsatile waveform. PVRs can provide valuable information about the presence of PAD, especially in cases in which the ABI may be artificially normal or elevated due to calcified vessels. An example of the noninvasive physiologic testing method is shown in Fig. 4 . Fig. 5 contains examples of normal and abnormal physiologic test results.
Noninvasive physiologic testing provides adequate information regarding the presence or absence of PAD, its severity, which segments are likely involved; however, further imaging is indicated when more specific information about the degree and exact location of PAD is needed, often in the case of planning before a procedure. The goal of imaging in PAD is to determine the location of disease (inflow vs outflow), whether stenoses or occlusions are confined to discrete or long segments, and severity of disease. Imaging may also help determine whether the patient is a better candidate for endovascular or surgical revascularization and may help in procedural planning. If endovascular revascularization is a consideration, further imaging can also help to provide information about the likelihood of long-term patency to assist the patient and the physician in the decision making process.
Arterial Duplex
Arterial duplex can be used as an adjunct to physiologic testing for assessing the presence and location of lower extremity PAD. Duplex testing is more costly and can be time-consuming. The technique is also dependent on the proficiency of the operator. Therefore this method is most often used to obtain focused information about the level and severity of arterial stenoses or occlusions, the patency of bypass grafts or stents, and the presence of pseudoaneurysms or arteriovenous fistulas. In the presence of stenosis, Doppler evaluation demonstrates elevated flow velocities within the stenotic arterial segment. Generally, at least a doubling of the velocity at the site of stenosis in comparison to a proximal normal segment indicates hemodynamically significant stenosis ( Fig. 6 ).
CTA and MRA
Computed tomography angiography (CTA) and magnetic resonance angiography (MRA) are considered highly sensitive and specific in the evaluation of PAD. CTA is inexpensive and quick, offering excellent visualization of inflow disease and surgical bypasses. However, CTA requires radiation exposure, although less than that required for catheter-based angiography. MRA, which is comparable to CTA in terms of inflow and surgical bypass visualization, is particularly useful for visualization of tibial vessels and also does not result in ionizing radiation exposure. MRA, however, is more costly than CTA and not as readily available; MRA is also contraindicated in patients with pacemakers or defibrillators. In addition, the risk of nephrogenic systemic fibrosis has been described in patients with Stages 4 or 5 chronic kidney disease who are exposed to gadolinium. Other limitations of MRA include difficulty with calcium visualization and inadequate assessment of peripheral stents due to artifact. For peripheral stent evaluation, CTA or arterial duplex is the preferred imaging modality.
Contrast Arteriography
Digital substraction arteriography (DSA) is often considered the gold standard for arterial imaging, with improved spatial resolution over CTA or MRA. DSA also offers the opportunity for endovascular revascularization during the diagnostic procedure. However, contrast toxicity, cost, and complications associated with arterial puncture make DSA difficult to use routinely as a diagnostic method for lower extremity PAD.
Medical management of PAD
The management of PAD should follow a 3-pronged approach: (1) identification and treatment of systemic atherosclerosis, (2) improvement of functional status, and (3) preservation of the limb. These therapeutic goals must be addressed simultaneously, as each has a significant impact on the long-term outcomes of patients with PAD, in terms of mortality benefit and improved quality of life ( Box 1 ).
Treatment of systemic atherosclerosis for cardiovascular risk reduction
Antiplatelet agents
Lipid-modifying agents such as 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase inhibitors to target low density lipoprotein cholesterol (LDL-C) less than 100 mg/dL (can consider target LDL-C<70 mg/dL in selected cases)
Treatment of blood pressure to achieve a target less than 140/90 or less than130/80 mmHg if concomitant diabetes or chronic kidney disease
Angiotensin-converting enzyme (ACE) inhibitors (or angiotensin receptor blockers)
Treatment of diabetes to achieve hemoglobin A1C goal of less than 7.0%
Smoking cessation
Improvement of functional status and quality of life
Cilostazol
Exercise training, preferably supervised
Diet and weight loss
Endovascular revascularization in selected cases
Surgical revascularization in selected cases
Preservation of the limb
Meticulous foot care
Treatment of diabetes to achieve hemoglobin A1C goal of less than 7.0%
Smoking cessation
Endovascular revascularization
Surgical revascularization
Treatment of Systemic Atherosclerosis for Cardiovascular Risk Reduction
Given the systemic nature of atherosclerosis, patients with PAD are at high risk for developing cardiovascular events such as myocardial infarction and stroke. Aggressive cardiovascular risk reduction should be a central component of the medical management of PAD.
Antiplatelet agents
Treatment with antiplatelet agents is recommended to reduce the risk of recurrent vascular events (myocardial infarction, ischemic stroke, or vascular death) in patients with lower extremity PAD. The data supporting this recommendation are mainly derived from the Antiplatelet Trialists’ Collaboration, a meta-analysis of 287 studies of antiplatelet agents. The meta-analysis included 135,000 patients who had clinical cardiovascular disease, including 9214 patients with PAD. In the PAD subgroup treated with antiplatelet therapy, there was a 22% odds reduction for adverse cardiovascular events, defined as myocardial infarction, stroke, or vascular death. The most common antiplatelet therapy used in these trials was aspirin. In comparing the efficacy of different doses of aspirin, the meta-analysis reported comparable proportional reductions in vascular events between 75 to 150 mg daily and 160 to 325 mg daily ranges. There was a significantly lower reduction in risk in those patients treated with less than 75 mg of aspirin daily. Current guidelines support the use of aspirin in daily doses of 75 to 325 mg to reduce the risk of cardiovascular events in patients with lower extremity PAD.
Clopidogrel, a thienopyridine drug, has been evaluated in the CAPRIE trial. This trial randomized approximately 19,000 patients with recent myocardial infarction, recent ischemic stroke, or moderately severe symptomatic PAD to 75 mg of clopidogrel daily versus 325 mg of aspirin daily. In the PAD subgroup, which consisted of 6452 patients with claudication and an ABI less than 0.85 or prior lower extremity bypass surgery, angioplasty, or amputation, those treated with clopidogrel had a 23.8% relative risk reduction for vascular events compared with those treated with aspirin. Based on the results of the CAPRIE trial subgroup analysis, current guidelines support clopidogrel 75 mg daily as an “effective alternative antiplatelet therapy to aspirin” in the prevention of vascular events in patients with lower extremity PAD. There is no current evidence to support the use of combination therapy with aspirin and clopidogrel for cardiovascular risk reduction in patients with lower extremity PAD.
Smoking cessation
Cigarette smoking is considered an important risk factor for the development of PAD, with a 3- to 6-fold increased risk of intermittent claudication in smokers versus nonsmokers. In addition, 1 study revealed that smokers with PAD developed claudication more quickly while walking than nonsmokers with PAD, and their leg symptoms took longer to subside. Although there have been no randomized controlled trials comparing outcomes in smokers versus nonsmokers with PAD, it has been demonstrated that smoking cessation in patients with PAD decreases the risk of future cardiovascular events and reduces progression to lower extremity CLI. In PAD patients who continue to smoke, the 10-year mortality has been reported to be as high as 40% to 50%, with most deaths due to stroke or myocardial infarction.
The approach to smoking cessation includes education, counseling, and the use of pharmacologic therapy. Several pharmacologic agents have been approved for use in smoking cessation. Nicotine replacement therapy (NRT) is a good first-line approach, given that the chemical addiction to nicotine is believed to drive patients to keep smoking. Nicotine replacement, which is considered safe in patients with vascular disease, can be achieved through several different modalities, including gum, spray, inhaler, or patch. Another pharmacologic agent is bupropion hydrochloride (Zyban), which has been shown to increase smoking cessation rates over placebo, from 12% in patients using placebo to 23% in those using bupropion.
Another agent, varenicline, has recently become available to aid in smoking cessation. Varenicline is a partial agonist of nicotinic acetylcholine receptors and has been shown in 2 randomized trials to be superior to bupropion and placebo in continuous abstinence rates at 12 and 24 weeks. However, concerns have arisen regarding the safety of varenicline, with a variety of reported adverse effects including falls, syncope, and vision disturbances. More importantly, the US Food and Drug Administration (FDA) recently advised health care providers that suicidal thoughts and aggressive behavior have also been reported in patients taking varenicline. Thus, varenicline must be used cautiously in patients who qualify for treatment, with increased vigilance on the part of physicians for the development of serious adverse effects. NRT can be prescribed in conjunction with bupropion but is not recommended with varenicline due to the mechanism of action.
Diabetes
Patients with diabetes mellitus have a 2- to 4-fold increased risk for developing PAD in comparison to patients without diabetes. One study revealed that the prevalence of PAD in diabetic patients was as high as 22.4% compared with 12.5% in patients without diabetes. This same study also demonstrated a high prevalence of PAD in patients with impaired glucose tolerance, up to 19.9% compared with 12.5% in patients with normal glucose tolerance. Patients with diabetes are also at increased risk for developing intermittent claudication and are more likely to develop infrapopliteal occlusive disease in comparison to nondiabetic subjects. Tight glycemic control has not been shown to reduce the incidence of intermittent claudication or rest pain. However, the UK Prospective Diabetes Study revealed that tight control of glucose decreases microvascular complications and may also decrease the risk of myocardial infarction. In addition, meticulous foot care is critical in PAD patients with diabetes, given their higher risk of developing limb ischemia and ulcerations.
Dyslipidemia
The NHANES study demonstrated that dyslipidemia is strongly associated with lower extremity PAD. Another study reported that the odds of developing PAD increase by approximately 10% for every 10 mg/dL increase in total cholesterol. The Heart Protection Study, which included 20,536 patients at high risk for cardiovascular events, including 6748 patients with PAD, randomized subjects to treatment with simvastatin (40 mg) versus placebo. Within the PAD subgroup, there was a 25% relative risk reduction in vascular events at 5 years in subjects treated with simvastatin, including in patients with normal LDL levels at baseline. The Scandinavian Simvastatin Survival Study demonstrated that subjects in the PAD subgroup treated with simvastatin had a lower likelihood of new or worsening claudication than those treated with placebo. Given that dylipidemia is considered a risk factor for developing PAD, and that treatment of dyslipidemia results in a reduction in major cardiovascular events in PAD patients, the National Cholesterol Education Program Adult Treatment Panel III has recommended similar lipid treatment strategies for patients with PAD as for those with CAD. As a result, current guidelines support the use of HMG CoA reductase inhibitor (statin) medications in patients with PAD with a goal LDL of less than 100 mg/dL. A target LDL of less than 70 mg/dL may be considered in PAD patients with multiple major risk factors including diabetes, poorly controlled risk factors such as current cigarette smoking, or multiple risk factors of the metabolic syndrome.
Hypertension
Hypertension is considered an independent risk factor for PAD. No data are available to confirm that treatment of hypertension reduces progression of lower extremity PAD or the incidence of intermittent claudication, though prior evidence supports treatment of hypertension to improve cardiovascular outcomes. For example, the Appropriate Blood Pressure Control in Diabetes trial included 950 patients with diabetes, 53 of whom had PAD defined by ABI less than 0.9. Subjects were randomized to moderate or intensive blood pressure treatment and followed for 5 years. The cardiovascular event rate in PAD patients receiving intensive blood pressure treatment was 13.6% compared with an event rate of 38.7% in those receiving moderate treatment; these results suggest that intensive blood pressure control has the potential of reducing cardiovascular events in PAD patients with diabetes. According to current guidelines, blood pressure should be managed to a goal of less than 140 mmHg systolic over 90 mmHg diastolic in all patients with PAD, with a further blood pressure goal of less than 130 mmHg systolic over 80 mmHg diastolic in patients who have concomitant diabetes mellitus or chronic kidney disease. Some concern was reported regarding the use of β-adrenergic antagonist drugs (β-blockers) for the treatment of hypertension in PAD patients based on previous case reports that β-blockers reduced lower extremity blood flow and led to an increase in claudication symptoms. However, subsequent studies have not reported any adverse events with the use of β-blockers; a meta-analysis of these studies concluded that β-blockers do not affect walking ability in patients with PAD.
Evidence suggests that ACE inhibitors may reduce the risk of cardiovascular events in patients with PAD beyond that expected from blood pressure control alone. The Heart Outcomes Prevention Evaluation (HOPE) study randomized 9257 high-risk patients to treatment with ramipril (an ACE inhibitor) versus placebo. Treatment with ramipril reduced the 5-year event rate (a composite of myocardial infarction, stroke, or vascular death) from 17.7% in the placebo group to 14.4% in the ramipril group. The benefit of ACE inhibition was also noted in the PAD subgroup, which included 4051 patients with PAD defined by ABI less than 0.9. The more recent Ongoing Telmisartan Alone and in combination with Ramipril Global Endpoint trial (ON TARGET) trial demonstrated that telimsartan, an angiotensin II receptor blocker, was equivalent to ramipril in prevention of cardiovascular events, including in the subgroup of patients with PAD. Current guidelines support the use of ACE inhibitors or angiotensin receptor blockers to reduce the risk of cardiovascular events in patients with lower extremity PAD.
Improvement of Functional Status and Quality of Life
Patients with intermittent claudication have reduced functional capacity and impaired exercise tolerance, often leading to difficulty in performing activities of daily living. Symptoms of claudication can range from mildly disabling to severely lifestyle-limiting, resulting in decreased quality of life. Effective therapy for intermittent claudication is essential to ensure improvement in the functional status of patients with lower extremity PAD, thereby directly enhancing their quality of life. Therapy for claudication includes supervised exercise training, pharmacologic therapy, and lifestyle modification. Supervised exercise training is the most important nonpharmacologic therapy for claudication and has been proven effective in improving pain-free and maximal walking distance in patients with PAD. Supervised exercise therapy is discussed in detail separately in this article.
Pharmacologic therapy: cilostazol
The main pharmacologic agent currently used in the treatment of claudication in patients with PAD is cilostazol, which received FDA approval in 1999. Cilostazol is a phosphodiesterase type 3 inhibitor which increases intracellular cyclic AMP concentrations. Cilostazol also inhibits vascular smooth muscle cell proliferation and platelet aggregation and causes vasodilation. However, neither platelet aggregation nor vasodilation is supported as the mechanism by which cilostazol improves claudication. Five prospective randomized controlled trials have been published to date examining the efficacy of cilostazol over placebo in improving claudication symptoms. The trials have revealed that cilostazol increases pain-free and maximal walking distance in PAD patients by 40% to 60% in comparison to placebo. A meta-analysis of these trials concluded that in addition to improving walking distance, cilostazol also improves health-related quality of life. The main side effects reported with cilostazol include headache, diarrhea, dizziness, and palpitations. Importantly, cilostazol is contraindicated in heart failure due to the previously observed increased mortality in long-term administration of oral phosphodiesterase type 3 inhibitors such as milrinone in patients with heart failure. A black box warning not to administer cilostazol to claudicants who also have heart failure of any severity is currently in effect.
Pharmacologic therapy: pentoxifylline
Pentoxifylline is a methylxanthine derivative that improves red cell deformability, has mild antiplatelet effects, and lowers plasma fibrinogen. Pentoxifylline was first approved in 1984 for the treatment of claudication based on earlier data that showed a modest improvement in maximal treadmill walking distance in patients treated with pentoxifylline versus placebo. Meta-analyses of the pentoxifylline trials revealed a small but marginal increase in walking distance with the use of pentoxifylline over placebo. A more recent study aimed at studying the efficacy of cilostazol versus pentoxifylline and placebo revealed that while cilostazol improved pain-free and maximal walking distance, pentoxifylline was no more effective than placebo in improving either pain-free or maximal walking distance. Therefore, based on available evidence, current guidelines suggest that although pentoxifylline may be considered in the treatment of claudication, it is unlikely to be of significant clinical benefit and its widespread use is therefore not recommended.
Pharmacologic therapy: other agents
Alternative pharmacologic agents and nutritional supplements have been studied in the treatment of claudication. Prostaglandins, which include prostaglandin E1 and oral analogs such as beraprost, have vasodilator properties and inhibit platelet aggregation. Although 1 randomized trial demonstrated that beraprost improved walking distance in patients with claudication, a more recent larger randomized trial found no benefit. Furthermore, beraprost was associated with significant side effects including flushing, headaches, and gastrointestinal effects. Given that available evidence has failed to show the effectiveness of prostaglandins in the treatment of claudication, it is unlikely that this class of drugs will be approved for this indication in the future. Angiogenic growth factors such as vascular endothelial growth factor have also been studied in the treatment of claudication, mainly because they have been shown to increase extremity blood flow and collateral formation in experimental models. However, further studies are needed to determine the efficacy of angiogenic growth factors in the treatment of claudication. Nutritional supplements such as l -arginine and gingko biloba have been studied but their efficacy in improving walking distance in patients with claudication is not well established. Vitamin E, which has also been previously studied, is not currently recommended as an effective therapy for claudication.
Revascularization for claudication
Current guidelines support the use of revascularization, whether endovascular or surgical, in selected cases only. Specifically, invasive revascularization for claudication should only be considered once the following factors have been taken into account: (1) response to at least 3 months of exercise or pharmacologic therapies for claudication has been inadequate; (2) a lifestyle-limiting disability due to claudication is present, preventing the patient from performing activities of daily living; (3) the vascular anatomy has been evaluated in terms of suitability for intervention, with a favorable risk to benefit ratio, such as in the case focal aortoiliac disease; and (4) the presence of other comorbid conditions that would otherwise limit the patient’s activity have been considered and evaluated before considering lower extremity revascularization. Patient preference also plays a significant role in the decision to proceed with interventional therapy for claudication. Patients should be referred early to a vascular specialist for evaluation and management and to determine if invasive revascularization is indicated.
Once patients have been appropriately selected for revascularization, further imaging with CTA, MRA, or contrast angiography may then be obtained to determine whether the arterial anatomy is more suitable for endovascular versus surgical revascularization. Endovascular revascularization is now used more frequently in the treatment of claudication due to rapid advancements in endovascular techniques, which include angioplasty with balloon dilation, atherectomy, and stenting. Long-term patency seems best in the endovascular treatment of iliac lesions, as compared with more distal lesions, which have lower patency rates over time; patency rates also decrease in the setting of long-segment occlusions, multiple tandem lesions, and poor run-off. Current guidelines recommend endovascular treatment as the preferred revascularization technique for short segment (3 cm or less) single lesions in the iliac or femoropopliteal segments. Surgical revascularization for claudication may also be considered, though it is not performed frequently for this indication in part due to the high cardiovascular risk for surgery in patients with PAD.
Lifestyle modification
Prior studies have shown that PAD patients with impaired lower extremity function demonstrate reduced physical activity levels in comparison to patients with normal lower extremity function, possibly contributing to subsequent disability. Given that patients with intermittent claudication are at the low end of the physical activity spectrum, they may be at increased risk for being overweight or obese. The result of this can lead to the development of the metabolic syndrome, a condition characterized by abdominal obesity, high triglyceride and low high density lipoprotein levels, hypertension, and glucose intolerance. It is particularly important to consider the possible negative consequences that the metabolic syndrome may have on peripheral circulation and ambulation in a population of patients already exhibiting significant limitations on physical functioning.
The metabolic syndrome can exacerbate the ambulatory dysfunction of PAD patients with intermittent claudication, leading to shorter walking distances and poorer health-related quality of life. One study of 423 patients with PAD and stable intermittent claudication revealed that PAD patients with the metabolic syndrome demonstrated shorter initial and absolute claudication distances compared with those PAD patients without the metabolic syndrome. Furthermore, patients with the metabolic syndrome exhibited shorter Walking Impairment Questionnaire (WIQ) distances, lower self-perceived health, and a lower health-related quality of life. Further studies are needed to test interventions designed to treat the risk factors of the metabolic syndrome, particularly insulin sensitivity, to improve physical functioning and long-term prognosis in PAD patients. The Diabetes Prevention Program revealed that exercise and modest weight loss can significantly impact insulin sensitivity and prevent progression to diabetes. There was a 58% reduction in the risk of developing diabetes in patients randomized to lifestyle changes with diet and exercise in comparison to the usual care group. It is reasonable to consider that in addition to preventing the development of diabetes, lifestyle modifications such as changes in diet, increase in daily exercise, and weight loss may also help in improving health-related quality of life in patients with lower extremity PAD, particularly those who are overweight and obese and at risk for developing the metabolic syndrome.
Preservation of the Limb
Meticulous foot care
A key strategy for limb preservation in patients with PAD is prevention of skin breakdown which can ultimately lead to ulceration and amputation. Prevention begins with meticulous foot care. Patients with PAD and concomitant diabetes should be evaluated regularly by a podiatry specialist. Patients should also be educated about performing daily foot inspections and using moisturizing creams regularly to prevent dryness. In addition, appropriate footwear should be used to prevent pressure-induced skin breakdown and ulceration.
Invasive therapies for CLI
CLI occurs when blood flow at rest is insufficient for tissue viability. Physical signs of tissue ischemia, such as dependent rubor and elevation pallor, calf atrophy, ischemic ulcers, or gangrene, should lead to rapid evaluation and consideration for lower extremity revascularization for limb salvage. It is estimated that up to 50% of patients with CLI will require revascularization for limb salvage and in those with disease that is not easily treatable invasively, up to 40% will require a major limb amputation within 6 months of presentation. Endovascular and surgical interventions are used in limb salvage, and selection of the specific revascularization strategy may depend in part on the vascular anatomy, the presence of inflow or outflow disease, and patient comorbidities.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






