Foot infections are a major cause of morbidity and mortality in diabetics. Evaluation of diabetic foot infections often requires clinical, radiologic, laboratory, and microbiologic assessment. Osteomyelitis has a profound impact on the prognosis and management of these infections, and diagnosis can be difficult; the gold standard remains bone biopsy. Despite a panoply of studies, the optimal management of diabetic foot infections remains poorly understood. Antibiotics, surgery, rehabilitation and/or off-loading, and glycemic control remain the cornerstones of treatment; alternative therapies remain largely unproven.
Foot infections rank high among the most feared and costly complications of diabetes mellitus. Levin and O’Neal point out that there are 21 million diabetics, 42 million diabetic feet, and 210 million diabetic toes in the United States, minus the amputated ones. In the United States, it is estimated that perhaps 5% to 10% of diabetics sustain foot infections each year. Diabetics have a 9- and 12-fold higher risk than their nondiabetic counterparts for developing cellulitis and osteomyelitis (OM) of the lower extremity, respectively. Annually an estimated 82,000 limbs are lost as a result of diabetes. Most diabetic foot infections (DFIs) are superficial; infection of deeper tissues occurs in about 25% of cases. It is estimated that 25% to 50% of all DFIs require minor amputation, whereas 10% to 40% require major amputation. Sixty percent of all amputations in diabetics are preceded by an infected foot ulcer. There is an increase in costs to patients, providers, and payers; of particular concern in at least 40% of patients undergoing major amputations is the loss in ability to live independently.
Diabetic patients have many risk factors predisposing toward foot ulceration and infection, including previous amputations, peripheral sensorimotor and autonomic neuropathies, resultant osteoarthropathy, abnormal biomechanical stressors, vascular insufficiency, wound healing deficits, and reduced mobility and vision. The impairment of neutrophil function in hyperglycemia and local hypoxic states also plays a major role. In 1 large cohort, independent clinical risk factors for the presence of infection in a diabetic foot ulcer (DFU) included penetration to bone, prior trauma, recurrent infection, duration of infection more than 30 days, and presence of peripheral vascular disease. Deep, recurrent, and multiple wounds are independently associated with development of OM. A wide variety of modalities for diagnosis and treatment are available, with variable amounts of data supporting their use. The authors attempt to review salient recent data regarding diagnosis and treatment of DFIs. It is important to note that not all ulcers represent infections, although the vast majority of DFIs originate from DFUs. Studies vary greatly in the definition of “cure,” but in general an infection may be “cured” without resolution of the underlying ulcer.
It should be noted that uninfected DFUs do not require antibiotic treatment. Multiple studies have considered the use of skin grafting, surgery, epidermal growth factors, hyperbaric oxygen, vacuum dressings, and other modalities for uninfected DFU therapy; treatment of these lesions is beyond the scope of this article.
Published guidelines
The Infectious Diseases Society of America (IDSA) issued extensive guidelines for the evaluation and treatment of DFIs in 2004. An update is now nearing completion; the interested reader should refer to this work for updated recommendations. The IDSA guidelines, which are accessible at http://www.journals.uchicago.edu/doi/pdf/10.1086/424846 , are recommended for their straightforward and pragmatic presentation of these complex issues. The International Working Group on the Diabetic Foot (IWGDF) published a similar set of guidelines in 2007 accessible at http://www.iwgdf.org/index.php and a recent set of proposed guidelines specifically for diabetic foot osteomyelitis (DFO).
Diagnosing infection
Initial Assessment
Despite the lack of clear consensus on numerous specific aspects of care for DFI, a general approach to care is commonly agreed upon. DFI must be diagnosed clinically and not based on the results of surface swab cultures. Signs of inflammation (local or systemic) in the presence of a DFU may suggest infection, although fever, leukocytosis, and elevated erythrocyte sedimentation rate (ESR) are present in less than 50% of cases.
The initial clinical evaluation of DFI, as suggested in the 2004 IDSA guidelines, mandates evaluation of the overall physiologic and psychosocial state of the patient, the biomechanical and neurovascular status of the affected limb, and the detailed anatomy of the ulcer itself ( Table 1 ). The infected area should be assessed for extent of local necrosis, presence of abscesses, and possible involvement of bone or joints.
| Level of Evaluation | Relevant Problems and Observations | Investigations |
|---|---|---|
| Patient | ||
| Systemic response to inflammation | Fevers, chills, sweats, vomiting, hypotension, tachycardia | History and physical examination |
| Metabolic state | Volume depletion, azotemia, hyperglycemia, tachypnea, hyperosmolality, acidosis | Serum chemistry analyses and hematologic testing |
| Cognitive state | Delirium, dementia, depression | Assessment of mental status |
| Social situation | Self-neglect, noncompliance, lack of home support | Interviews with family, friends, health care professionals |
| Limb or foot | ||
| Biomechanics | Deformities (Charcot, claw/hammer toes, calluses) | Clinical examination and radiography (≥2 images) |
| Vascular Status | ||
| Arterial | Ischemia, necrosis, gangrene | Foot pulses, ABI, TcP o 2 , duplex ultrasonography, angiography |
| Venous | Edema, stasis, or thrombosis | Physical examination and duplex ultrasonography |
| Neuropathy | Loss of protective sensation | Light touch, microfilament pressure, or vibration perception |
| Wound | ||
| Size and depth (tissues involved) | Necrosis, gangrene, foreign body, and involvement of deeper tissues | Inspect, debride, and probe the wound; radiography (≥2 images) |
| Presence, extent, and cause of infection | Purulence, warmth, tenderness, pain, induration, cellulites, bullae, crepitus, abscess, fasciitis, and osteomyelitis | Gram stain, culture of deep tissue, imaging (see text) |
After establishing the presence of a DFI, the patient should first be assessed for the presence of any signs of systemic response to infection, as well as the metabolic state. This will help triage the patient, helping the clinician determine the level of urgency required in addressing the infection, and determining the best venue for care. On presentation, the wound should be specifically assessed for the severity and extent of the infection.
The severity of a DFI dictates the initial course of treatment, including selection of antibiotic, route of administration, and duration of treatment, as well as the need for hospitalization. The IDSA and the IWGDF guidelines present similar classification schemes of infection severity ( Table 2 ). These schemes were recently validated in a longitudinal study of 1666 diabetic patients, which found that with an increasing class of severity, there was a significantly increased risk for amputation, higher-level amputation, and lower-extremity hospitalization.
| Clinical Description | IDSA | IWDGF |
|---|---|---|
| Wound without purulence or any manifestations of inflammation | Uninfected | 1 |
| ≥2 Manifestations of inflammation (purulence or erythema, pain, tenderness, warmth, or induration); any cellulitis or erythema extends ≥2 cm around ulcer, and infection is limited to skin or superficial subcutaneous tissues; no local complications or systemic illness | Mild | 2 |
| Infection in a patient who is systemically well and metabolically stable but has ≥1 of the following: cellulitis extending 12 cm; lymphangitis; spread beneath fascia; deep tissue abscess; gangrene; muscle, tendon, joint, or bone involvement | Moderate | 3 |
| Infection in a patient with systemic toxicity or metabolic instability (eg, fever, chills, tachycardia, hypotension, confusion, vomiting, leukocytosis, acidosis, hyperglycemia, or azotemia) | Severe | 4 |
OM is often missed in the absence of inflammatory signs, but is present in approximately 20% of all DFI and complicates its management more than any other single factor. Acute Charcot arthropathy or gout is a noninfectious cause of foot inflammation that can mimic DFO. OM serves as a very poor prognostic marker, greatly increasing the likelihood that the patient will ultimately require amputation. In the rest of this article, the management of diabetic foot complicated by OM is discussed separately from soft-tissue DFIs.
Microbiology of the Diabetic Foot
As opposed to normally sterile body sites (bladder, CNS, intravascular space), ulcerated and normal skin are colonized by a varied range of microorganisms, of which a limited set (eg, gram-positive cocci such as Staphylococcus aureus and Group B streptococci, and gram-negative bacilli such as Klebsiella and Pseudomonas) can act as pathogens. Although newer molecular methods show the diversity of fastidious flora in most DFI, the clinical utility of identifying such organisms is not clear. Normal skin, as opposed to ulcerated skin, has an ability to limit the residence and invasion of common skin pathogens. Infection is distinguished from colonization of such tissue by the presence of local tissue destruction and inflammation—not by the presence or absence of pathogenic bacteria.
Pathogens causing DFIs and OM are the same, although S. aureus is proportionally overrepresented in OM (in 1 study, S. aureus 40%, Enterobacteriaceae such as Escherichia coli or Klebsiella pneumoniae 40%, streptococci 30%, S. epidermidis 25%). Specific syndromes associated with particular bacteria include macerated ulcers after soaking ( Pseudomonas ) and “fetid foot” (a gangrenous and/or necrotic malodorous foot as a result of mixed aerobic and/or anaerobic infection). More commonly, a patient will present with a DFI that is antibiotic-naïve (wherein gram-positive organisms predominate) or previously treated (wherein resistant gram-negative bacteria become likelier, with increased antibiotic resistance seen after broader and longer courses of antibiotics). Among the few published cases of fully vancomycin-resistant S. aureus (VRSA), dysvascular patients have been highly represented (so far, VRSA has been found in 1 diabetic foot ), highlighting the need for vigilance and antibiotic stewardship in DFI management.
Wound Cultures
A swab culture of the surface of a DFU usually reveals at least 1 potential pathogen; such surface cultures can be difficult to use in guiding treatment because they do not distinguish a colonized ulcer from an infected one. Culturing an uninfected DFU is rarely necessary (except in limited cases when screening for resistant colonizing flora). Swab cultures of the surface of an infected DFU often fail to represent the underlying pathogen. When deep DFIs are suspected, deep cultures are most suitable for guiding antibiotic therapy.
In most cases, cultures of DFI should be obtained before the start of treatment (although a recent study found a striking lack of association between receipt of prior antibiotics and bone culture positivity). When cultures are obtained, the technique used to obtain tissue for culture and the type of tissue taken strongly affect the validity of the result. Ideally, deep cultures should be obtained without traversing the wound bed, to avoid the potential contamination of deep tissue with surface colonizing flora. After removing overlying necrotic debris, specimens should be obtained from the wound base or deeper tissues. It is important not to rely upon cultures from undebrided wounds or from wound drainage, whose correlation with deep cultures is poor. Blood cultures should be obtained in systemically ill patients. Aerobic and anaerobic bacterial cultures are sufficient in most cases to yield a diagnosis; fungi, mycobacteria, and viruses are uncommon causes of DFI.
New molecular methods attempt to determine whether cultured isolates are colonizers or pathogens by identifying specific virulence factors. Although these strategies are intriguing, their clinical utility remains unproven at this point.
Diagnosis of Diabetic Foot Osteomyelitis
In many cases of DFI, presence of infection is evident but severity and extent are not ( Fig. 1 ) . The need for surgery, the duration of antibiotics, and the outcome all depend strongly on whether the underlying pedal bone is infected. Because diabetic peripheral neuropathy predisposes to Charcot disease, which can mimic OM, distinction of infected and uninfected bone is usually difficult.
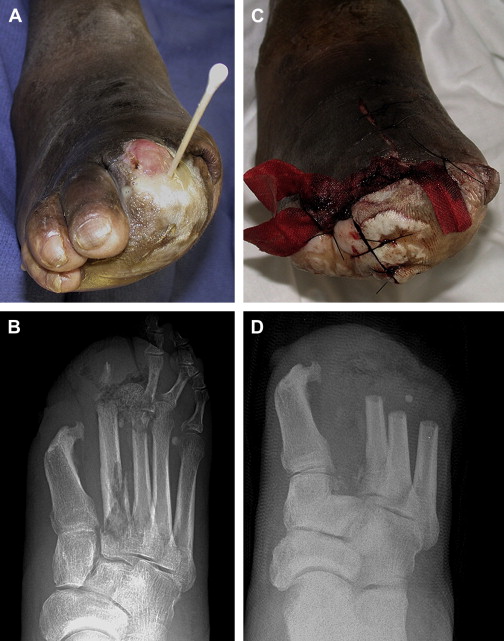
Physical examination
Physical examination plays a key role in the assessment of OM in DFI. DFO is typically defined as contiguous spread of infection from the soft tissue to bone, through the cortex, into the marrow space. The possibility of OM should be considered in all DFU, but particularly in larger (>2 cm), deeper (>3 mm), and more chronic ulcers. Swollen (“sausage”) toes and elevated ESR further suggest OM. The probe-to-bone test has a high positive predictive value in patients with suspected DFO. When exposed bone is observed, or a metal probe advanced into the ulcer comes in contact with bone (where it produces a characteristic feel), OM is likely (sensitivity 60%, specificity 91%).
Laboratory testing
Leukocytosis is generally a poor marker for OM. ESR and C-reactive protein tests on their own are inadequate to diagnose OM, but when coupled with clinical assessments (ie, ulcer size) their utility is somewhat augmented.
Imaging for osteomyelitis
Plain x-rays should be ordered for most patients with a DFU, despite sensitivity for DFO around 54%. Observable radiographic changes generally do not occur in the first 2 weeks of OM, which may explain in part the lack of sensitivity observed in clinical studies. Early changes classically seen in OM on x-ray imaging include periosteal reaction, focal osteopenia, or erosions; sequestrum, or islands of necrosed bone, can be seen in later chronic OM. Because the sensitivity of x-rays increases substantially with time, stable patients with suspected OM and negative initial plain films can be reimaged 2 weeks later.
Advanced imaging
Studies of advanced imaging techniques in diagnosing DFO have variable results; as a general observation higher pretest likelihoods of OM enhance the evaluated performance characteristics of these tests. Bone scans are reasonably sensitive (approximately 80%–85%) but nonspecific (approximately 30%–40%). Indium-111–tagged leukocyte scans offer somewhat improved specificity (75%). Other radiolabeling techniques include sulesomab and Tc-dextran scintigraphy which have yet to become common. High-resolution ultrasound and PET scanning also show promise, but few data are available.
Magnetic resonance imaging (MRI) is the imaging procedure of choice when OM is suspected, but not confirmed, by initial examination. MRI offers superior sensitivity and specificity (approximately 90% and 80%, respectively). Defects in soft tissue can be visualized along with infected bone. MRI-based diagnosis of OM can be confounded by false positive results (from Charcot disease) or false negative results (in early OM).
Bone biopsy
In contrast to laboratory and radiologic testing, bone biopsy provides a definitive diagnosis of OM. Studies of DFO and other types of chronic lower extremity OM convincingly show the limitations of shallow cultures for the identification of deep pathogens. The oft-cited caveat to this recommendation is the presence of a monomicrobial S. aureus culture from a draining sinus, which correlates highly with the presence of S. aureus in the bone culture.
Bone biopsies should be performed without traversing the open ulcer, using surgical or interventional radiographic approaches, to avoid contamination with surface flora. Open bone biopsies during surgery or debridement are more easily obtained, but may be more commonly contaminated by ulcer microflora.
Bone biopsy specimens should be sent for histopathology and bacterial culture (both aerobic and anaerobic). Histopathology can show osteitis or osteonecrosis that provide a definitive diagnosis. A positive culture result provides an opportunity for targeted antibiotic selection, with resultant decreases in the risk for adverse events, selection of resistant flora, and cost. Bone biopsy is most useful in cases where the diagnosis remains in question, or when the patient’s previous culture results or antibiotic therapy require a specific microbiologic diagnosis.
Treatment
General approach
In principle, the treatment of a diabetic foot seems to be straightforward, with clear goals: stabilization of the patient, control of the infection, prevention of infection-related morbidities, and preservation of function. An initial treatment plan of a DFI is often a 2-pronged attack of antibiotic therapy and surgical intervention of varying intensity; and it includes decisions regarding wound dressing, off-loading, and close, systematic follow-up by a multidisciplinary team, which includes infectious disease specialists, surgeons, endocrinologists, podiatrists, and physiatrists. Unfortunately, the optimal approach to many aspects remains unclear, in spite of innumerable studies focusing on the care of DFIs.
Factors complicating the establishment of an optimal approach
The treatment of DFIs has been the subject of countless clinical trials and publications over the past decades. Despite the surfeit of data, the goal of establishing an optimal, evidenced-based approach to the management of this problem remains beyond reach. Several factors contribute to this situation. The vast majority of clinical trials have been noncomparative and nonrandomized. The terms used in recent history to describe DFI severity, extent, and outcome have not been standardized. There is little agreement as to what even constitutes an important outcome measure. Thus drawing meaningful conclusions from the collective experience represented in the literature proves challenging.
Compounding these issues is the fact that, when controlled clinical trials have been conducted, many of the attempts at designing them have been hampered by small size, poor design, and lack of replication. Underscoring this problem is a recent systematic review of controlled clinical trials assessing antimicrobial interventions for DFUs. Of 1903 identified trials, only 23 were deemed to be of sufficient quality to contribute meaningful results.
Venue of treatment
One of the first decisions faced by the clinician treating a diabetic wound infection is determining if hospitalization is required. In most instances, patients with mild infections can be treated in the outpatient setting with oral antibiotics and close clinical follow-up. Very few studies have assessed the efficacy of outpatient antibiotic management of DFIs. The largest such study was an Italian multicenter observation trial of 271 patients treated for DFIs in the outpatient setting. A cure or improvement was documented in 93.4% of those enrolled, reflecting the results of previous smaller studies. Of note, approximately 60% of the patients in this study received parenteral outpatient antibiotics.
Patients who meet the criteria for severe infections almost always require hospitalization. Moderate DFI cases need to be assessed on a case-by-case basis; in the absence of complicating features, these patients can generally be treated as outpatients. However, a number of factors need to be considered, including the management of metabolic instability and pain. Often, hospitalization is prompted by the need for diagnostic tests or intravenous antibiotics when they cannot be arranged on an outpatient basis in a timely manner. The complexity of dressing changes and the ability of a patient to comply with treatment also need to be assessed when considering the need for admission.
Antibiotic choice
There is little evidence from randomized clinical trials to guide antibiotic selection or duration. There is even controversy over which bacteria cultured from the wound require specific treatment. No specific regimen has found to be more effective than others. Many different antibiotics have the potential to treat most common bacteria that cause DFIs. Therefore, an appropriate empiric regimen must be selected from an array of antibiotics, guided by clinical severity and local antibiotic resistance patterns. There is little evidence to suggest that targeting all isolated pathogens in a polymicrobial culture is necessary. This point is underscored by the results of several recent studies evaluating newer agents in the treatment of DFIs. Monotherapy with relatively narrower-spectrum antibiotics (linezolid, daptomycin, ertapenem, and moxifloxacin) was equivalent to monotherapy with broader-spectrum comparator antibiotics, even when organisms resistant to the antibiotic regimen were isolated.
Despite the lack of a preferred regimen for DFIs, treatment guidelines agree that targeting S. aureus is paramount. S. aureus is the most common cause of DFI, and empiric antibiotic coverage should always include an antistaphylococcal agent. In the absence of risk factors for methicillin-resistant S. aureus (MRSA), the frequent use of first generation cephalosporins and antistaphylococcal penicillins needs to be assessed carefully. Nevertheless, it is interesting to note that 2 large, well-conducted randomized controlled trials found that in patients with polymicrobial wound infections containing MRSA, receipt of vancomycin was not associated with a change in clinical outcome. Despite these findings, MRSA should not be ignored when isolated from a DFI; rather these findings again show the difficulty in determining the pathogenic role of specific organisms. In general, less virulent gram-positive organisms (coagulase-negative staphylococci and diphtheroid species) do not need to be directly treated. Enterococcus , another gram-positive organism, does not typically require targeted therapy when isolated in polymicrobial infection.
Empiric treatment of aerobic gram-negative organisms (ie, K. pneumonia e, E. coli , and Proteus species) should be restricted to patients presenting with severe DFI or those with chronically infected diabetic wounds with prior antibiotic exposure. The specter of Pseudomonas looms large when selecting an empiric regimen of antibiotics for moderate to severe DFIs. Determining when to target Pseudomonas remains unclear. It is commonly found as part of a polymicrobial infection, but its role as a pathogen in this setting is difficult to determine. Some experts suggest that treatment does not need to be directed at Pseudomonas unless it is the predominant organism isolated from a deep tissue specimen, or if the patient presents with sepsis. The growing prevalence of extended spectrum beta-lactamase E. coli and K. pneumoniae in DFIs is of great concern. As these organisms become more common, the approach to empiric antibiotic therapy for DFIs will become increasingly complicated.
This difficulty in deciding whether to target a cultured organism also clouds the treatment of obligate anaerobes, like peptococci, peptostreptococci, and Bacteroides species. These organisms, too, can often be isolated from polymicrobial infections; their overall contribution to the infection is likely to be minimal. Only when there is severe necrosis or gangrene do obligate anaerobes need to be specifically targeted.
Duration and route of therapy
As with choice of antibiotics, the duration of therapy for DFIs has not been systematically studied. Current guidelines suggests that 1 to 2 weeks of oral therapy are adequate for mild DFI, whereas 2 to 4 weeks of oral therapy should be administered for moderate and severe cases as long as there is no associated OM. The approach to DFO is discussed in the following section.
In spite of these seemingly clear recommendations, it is often difficult and possibly risky to determine a priori the exact duration of treatment. The optimal length of antibiotic therapy in a given individual is dependent on a substantial range of variables. Removal of necrotic tissue and pus, and selection of antibiotics with advantageous pharmacokinetics, can significantly reduce the total duration of antibiotics required. Antibiotic concentrations achieved in the infected tissue may affect outcome. Common sense suggests that in a patient with a severely impaired vascular supply to the infected foot, the ability of antibiotics to reach their target will be hindered. Most studies evaluating this scenario have been small; some report decreased levels of antibiotics in ischemic tissues despite adequate serum levels, but others have found the opposite.
The need for flexibility and common sense in deciding whether to stop or continue antibiotic therapy is emphasized in the existing guidelines. Both sets of guidelines present algorithms to assist clinicians with assessing for an inadequate response to treatment. Ultimately, careful follow-up is essential in determining when to stop antibiotics.
Topical antibiotics
Topical local administration of antimicrobials to DFI has several potential advantages, such as increased target site concentration, avoidance of systemic toxicity, and preservation of normal bowel flora. In addition, some potentially effective agents (ie, mupirocin, pexiganan) can only be administered topically. Regardless of their benefits, the role of topical antimicrobial or antiseptic therapy is limited to mild infection; these agents do not penetrate tissue far beyond their site of application and are therefore inappropriate for deeper infection. Certain topical agents may in fact inhibit wound healing on a cellular level.
Until very recently, no topical agent had been shown to be effective in any clinical trials. In 2008, a randomized controlled trial of more than 800 patients evaluated the efficacy of pexiganan, an antimicrobial peptide, in comparison to ofloxacin for the treatment of mildly infected DFIs. It showed equivalent outcomes in regards to overall clinical improvement, microbiological eradication rates, and wound healing rates.
Determination of the need for surgery
The role of surgery in DFIs is dual: the first goal is to control deep infection and the second is to salvage the foot. Many DFIs present with an obvious need for surgical intervention, ranging from simple debridement to emergent amputation in the setting of critical limb ischemia or necrotizing fasciitis. A systemically ill patient may not respond positively despite appropriate antibiotics until the infection has been adequately debrided.
Despite the many obvious benefits of surgical intervention, increased risk for morbidity may exist. Deformities created by surgery can have deleterious long-term effects on wound healing as well as the function of the limb. A surgeon must consider the blood supply to the remaining tissue, the effect of the proposed surgery on biomechanical function, and the optimal approach to wound closure.
Early surgical intervention has been shown to decrease the need for eventual amputation, decrease the time needed for return to function, and to reduce the duration of antibiotics. Patients with noncritical ischemia can generally be treated without revascularization. In the setting of critical ischemia, early revascularization (within 24–48 hours) is advisable to avoid amputation although sepsis may lead to unavoidable delays. However, much like the evidence guiding antibiotic management, the body of literature pertaining to the best surgical approach to DFIs is lacking in consistent conclusions. Comparative and randomized studies of surgical treatment, using consistent terminology and techniques, are lacking. Although the benefits of surgery in the short-term can be self-evident, very few studies have reported the long-term outcome of surgery in the infected diabetic foot.
Diabetic foot osteomyelitis
The approach to management of DFO is highly controversial. Despite the frequent occurrence and high morbidity of DFO, published literature provides little guidance as to the optimal approach to treatment. It is important to understand that DFO is generally a result of contiguous spread of infection from overlying tissue. Often, the presence of OM can be difficult to detect clinically. As a result DFO is typically chronic when it is finally identified. Unlike acute hematogenous OM, for which a 4- to 6-week course of antibiotics is typically curative, chronic OM (in nondiabetics as well as diabetics) has historically required antibiotics and surgical debridement of devitalized bone. Opinions vary on the necessity and timing of surgical treatment. Some studies recommend aggressive early surgery in almost every patient. Conversely, a growing body of literature reports successful treatment with antibiotic therapy and surgical debridement in certain selected patients. As a result, there is no clear standard of care for the management of DFO. Current guidelines allow that in some cases, antibiotic therapy alone can be sufficient to achieve cure in DFO, but predicting which patients will ultimately fail medical management cannot be done with any certainty.
Several factors complicate the choice of antibiotics in DFO. Adequate antibiotic penetration of infected bone historically mandates the use of intravenous preparations, but the availability of several highly bioavailable oral antibiotics question the necessity of this practice. Treatment duration in DFO is often long, increasing the risk for antibiotic-related side effects, but the optimal duration of antibiotic is not well understood. Although treating susceptible organisms with antibiotic monotherapy is usually sufficient, exceptions exist (ie, quinolones or rifampin as monotherapy for S. aureus ). The IDSA guidelines suggest basing the duration on the extent of residual viable and necrotic tissue after debridement: if all necrotic tissue is successfully removed, treatment can be as short as 2 to 5 days. In the presence of residual infected or necrotic bone, there is no clear endpoint to antibiotic therapy.
Adjunctive modalities
Several other modalities have been employed to treat DFIs. These include negative-pressure wound healing (eg, V.A.C. dressings, KCI, San Antonio, TX, USA), hyperbaric oxygen therapy, granulocyte colony stimulating factor (GCSF), and maggot debridement therapy (MDT). These modalities have most often been studied in noninfected DFU, or in some cases in DFI following surgery. As is the case with most aspects of diabetic foot management, case series and anecdotal reports comprise most of the current body of evidence evaluating these treatments; definitive evidence for their efficacy is lacking. The IDSA guidelines do not address the use of these adjunctive therapies for DFIs. In the more specific setting of DFO, the IWGDF recommendations for DFO state simply “there is no evidence to support the use of hyperbaric oxygen, granulocyte colony stimulating factor (G-CSF) or larval therapy in the treatment of diabetic foot osteomyelitis.” Negative-pressure wound healing is not addressed.
Negative-pressure wound healing was found to be most likely safe and beneficial in a subset of patients in a recent review of the few randomized controlled trials available. In 2 of the larger studies evaluating any adjunctive therapy, benefit has been found in postsurgical patients recovering from a transmetatarsal amputation in the setting of DFO and the treatment of noninfected diabetic wounds, as compared with standard wound dressing. DFI not managed with transmetatarsal amputation has not been systematically studied.
Hyperbaric oxygen therapy was found to lead to a significant reduction in the need for major amputation and improved healing at 1 year in a recent systematic review. The number of patients enrolled in the available studies was small, however, and design flaws limited the studies’ usefulness.
A recent meta-analysis of GCSF therapy analyzed data from 167 patients and found a significant reduction in surgical intervention in the treatment arm, including amputations. There was also a tendency toward decreased antibiotic use. Larger scale studies are required.
MDT is a simple, efficient method to separate necrotic tissue from living tissue, typically using larvae of the fly Lucilia sericata . It has been used in cases of DFI not responding to conventional therapy, but randomized controlled trials do not yet exist.
Summary
A multidisciplinary approach to postinfection wound management, with off-loading, intensive rehabilitation, and a wound care program, improves outcomes. Control of diabetes and vasculopathy is important to enhance wound healing and prevent recurrences. Treatment leads to a good clinical response in 80% to 90% of mild to moderate infections and 60% to 80% of deeper infections, including OM. Relapse occurs in 20% to 30% of cases, notably in those with OM or poor vascular supply.
DFIs, a major cause of morbidity, mortality, and health care expenditure in diabetics, are common and frequently pose difficult diagnostic and therapeutic problems. Although new studies slowly advance our knowledge of optimal therapy, the evidence base remains incomplete. Diagnosis requires careful physical examination, often coupled with data from radiology, microbiology, pathology, and the laboratory. A multidisciplinary approach with careful antibiotic selection, surgery in selected cases, and attention to physiologic and biomechanical parameters of healing remains the cornerstone of treatment. New techniques, and refinements of current methods, will lead to better care for these devastating and costly infections.
No funding source to be acknowledged.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






