CHAPTER 52 Unlinked Arthroplasty
PART A Distal Humeral Hemiarthroplasty
INTRODUCTION
Distal humeral hemiarthroplasty (DHH) was first described in 1947, yet over the past 60 years there have been only a few small series reporting on this technique.1,14,17,25–28 Early series reported treatment of a variety of pathologic conditions, including trauma, inflammatory arthritis, and tumor. Prostheses included both custom-made (of various materials) and nonanatomic humeral components from total elbow systems (capitellocondyllar, kudo) and anatomic components (Street). These early surgeries met with mixed results. The past decade has witnessed renewed interest in DHH with advancements in our understanding of elbow biomechanics and with growing experience in the difficulty of achieving effective outcomes in treating complex distal humerus fractures. This technique provides a treatment alternative that bridges the gap between internal fixation and total elbow arthroplasty (TEA) in unsalvageable distal humeral articular surface pathologies.
INDICATIONS AND CONTRAINDICATIONS
The indications for DHH are evolving, both in terms of what is achievable and what is appropriate (Table 52-1). Initial experience has been in the younger patient or the physically active older patient with a comminuted intra-articular distal humeral fracture (sometimes combined with a column fracture) that involves both trochlea and capitellum, where an adequate reduction and internal fixation cannot be achieved. Open reduction internal fixation (ORIF) remains the gold standard of operative treatment; however, patient factors and bone architecture may limit the surgeon’s ability to reconstruct the elbow. Series reporting on ORIF of comminuted intra-articular fractures indicate a substantial rate of unsatisfactory outcomes and a significant complication rate in complex cases.1,14,17,23,24
TABLE 52-1 Indications and Contraindications for Humeral Hemiarthroplasty
| Indications | Contraindications |
|---|---|
Insufficient bony column support, especially epicondyle condylar comminution resulting in instability |
ORIF, open reduction and internal fixation.
Total elbow arthroplasty has gained acceptance as a primary treatment option for intra-articular distal humeral fractures in lower demand, elderly patients with osteopenic bone.4,9–11,20,22 Its application to younger active patients is attendant with the risk of early prosthesis failure.
PRINCIPLES OF DISTAL HUMERAL HEMIARTHROPLASTY
Stability of the intact elbow joint is provided by ulnohumeral congruity,18 a competent radiocapitellar articulation, and collateral ligament integrity.5 Because DHH is an “unlinked” reconstruction, the prosthesis must have a high degree of congruency with the radius and ulna to provide stable force transmission through both medial and lateral articular columns; therefore, sizing is important. For the DHH to function optimally, the prosthesis must be oriented such that the elbow rotation axis relative to the insertions of the anterior band of the medial collateral ligament (MCL) and lateral ulnar collateral ligament (LUCL) is restored.3 This ensures that the ligaments remain isometric through a functional range of motion. The proper joint rotation axis can be determined by surface landmarks, medially at the anterior inferior surface of the medial epicondyle and laterally at the center of a circle formed by the articular surface of the capitellum.2,5–8,19,21
IMPLANT CONSIDERATIONS
There are currently two systems available (and an additional one, Coonrad/Morrey [Zimmer, Warsaw, IN] under development) that allow DHH with a prosthesis based on normal articular geometry (Fig. 52-1): (1) the Sorbie Questor elbow system (Wright Medical Technologies, Arlington, TN) and (2) the Latitude elbow system (Tornier, Stafford, TX). All require cement fixation and, because they may be subjected to significant forces, optimal cementing techniques should be used.7 The Sorbie Questor humeral component is a monoblock anatomic prosthesis with three sizes (small, medium, and large) that allow a “best fit” in 95% of all elbows.29 It allows fixation of comminuted bone fragments of either supracondylar column. There is no anterior flange or long-stem option to augment humeral fixation. This system allows conversion only to an unconstrained TEA, which may not address future instability issues. Mechanical studies of the Sorbie humeral component indicate that an intact radial head is necessary for elbow stability despite an intact MCL.13 It is attractive because it removes little bone and is relatively easy to insert.
PREOPERATIVE PLANNING
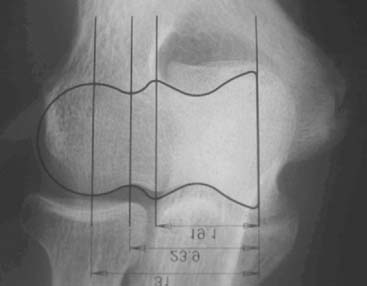
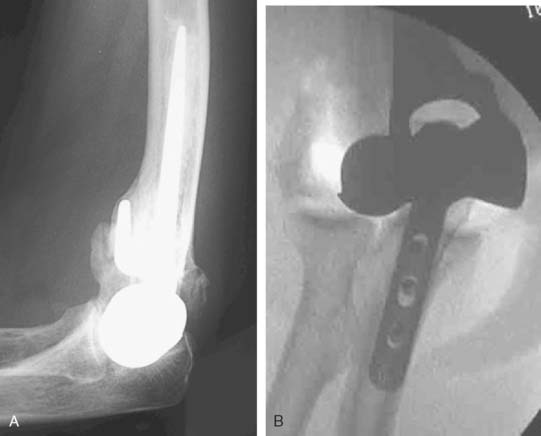
Preoperative imaging studies should include anteroposterior (AP) and lateral views of the elbows. Plain radiographs of the contralateral elbow permit additional templating of the distal humerus for appropriate prosthesis sizing. Sizing is based on the direct correlation between the AP dimensions of the normal articular surface and the radii of the capitellum and trochlea (Fig. 52-2).26 Preoperative assessment might also include a fine-cut computed tomography (CT) scan to evaluate articular comminution, column involvement, and associated injuries to the radial head and coronoid (Fig. 52-3).
OPERATIVE TECHNIQUE
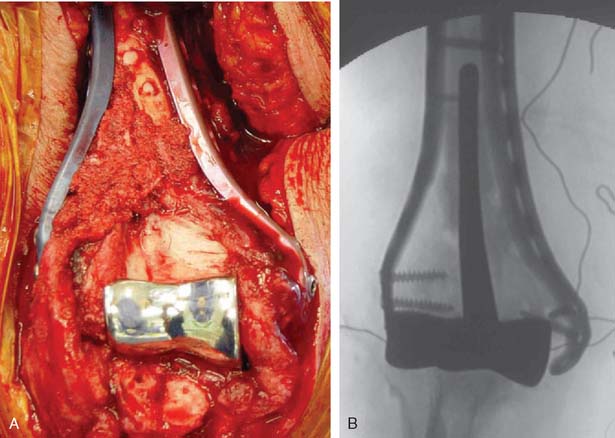
The patient is positioned in the lateral decubitus position. A tourniquet is applied and the arm secured to a short arm support. This allows flexion of the arm to 120 degrees, which facilitates insertion of the component. This also places the posterior aspect of the distal humerus parallel to the floor, when the elbow is flexed 90 degrees. A large sterile basin is placed beneath the arm to catch any bone fragments or instruments. The elbow is approached through an extensile posterior incision, and thick subcutaneous flaps are developed to provide access to both epicondyles and columns. The ulna nerve is exposed and protected. A Chevron osteotomy of the olecranon is performed at the level of the sigmoid fossa bare area, and the triceps mechanism is reflected sufficiently proximal to expose any supracondylar fracture extension (see Chapter 7). “Triceps-on” exposures provide poorer exposure and are associated with increased joint instability. During exposure, the humeral insertions and origins of the MCL and LUCL must be preserved.
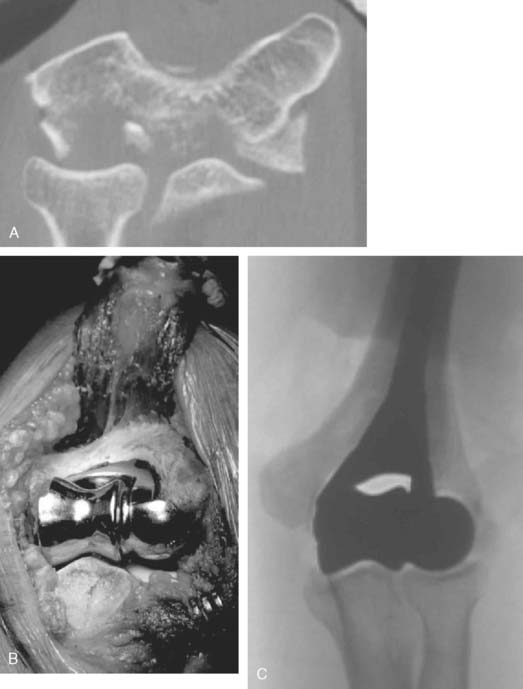
Once exposed, the articular surface can be inspected. If the combination of fracture comminution, osteopenia, and patient considerations favors DHH then trial components should be templated with the radial head and anterior sigmoid fossa to confirm proper fit and to avoid overstuffing of the joint. Injury to the radial head, coronoid, and collateral ligaments must be identified to proceed with this option and to ensure a stable reconstruction (see Fig. 52-3).
Next, the bone cuts must be made so that the prosthesis can be inserted to the proper depth and rotation to recreate the flexion axis. Free-hand cuts are generally preferred because bone loss from fracture comminution complicates accurate placement of the cutting jigs.
The trial prosthesis of appropriate size is inserted. Strict attention should be paid to the depth of insertion and rotation when the trial is seated because this determines ligament tension. Once the trial is in place, the ulna is reduced over its articular surface and the joint taken through a range of motion. Difficulty reducing the ulna suggests that the flexion axis is too distal, and the humeral cuts should be revised to prevent an overly tight reconstruction. The tracking of the anterior half of the olecranon during elbow flexion and extension can also provide a sense of the correct prosthesis rotation (Table 52-2).
TABLE 52-2 Pitfalls and Complications of Hemiarthroplasty
| Problem | Cause | Solution |
|---|---|---|
| Flexion contracture (intraoperative) | ||
| Joint translocation | Reassess ligament repair | |
| Rotatory instability | ||
| Block to flexion | ||
| Radiocapitellar mismatch | ||
| Olecranon osteotomy nonunion | ||
| Ulna neuritis | ||
| Painful joint | ||
| Stiff elbow |
EUA, examination under anesthesia; LCL, lateral collateral ligament; ORIF, open reduction and internal fixation; PLRI, posterolateral rotatory instability; TEA, total elbow arthroplasty.
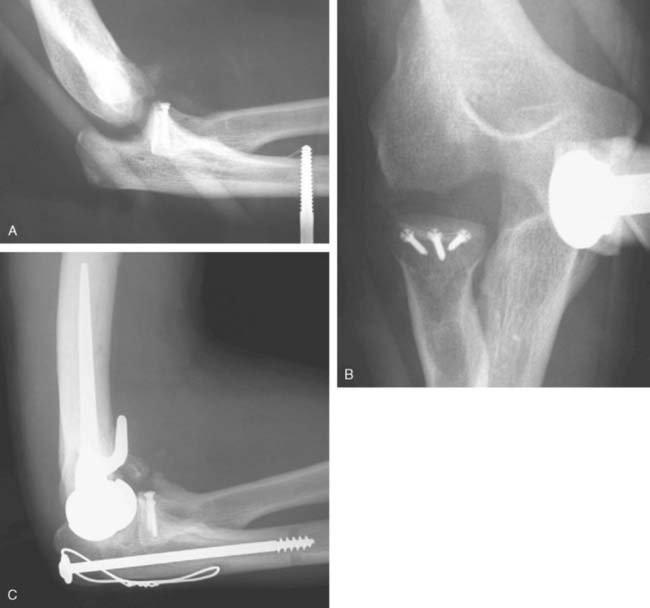
A cement restrictor should be used to provide good pressurization of the antibiotic cement. The final prosthesis is then inserted to the correct position. The ulna is reduced to the distal humeral prosthesis, and the elbow is extended (not cycled) until the cement is cured. The olecranon osteotomy is fixed using a tension band or a periarticular olecranon plate and always grafted with autologous bone from the trochlea (Fig. 52-4). Although the plate provides the best fixation (and therefore allows more aggressive early mobilization), there are times when soft tissue considerations prevent its use. The elbow should then be taken through a range of motion to ensure the following: (1) motion has not been constrained by an overly tight reconstuction; (2) the olecranon osteotomy remains reduced as tension develops in flexion; and (3) the ulna nerve does not develop undue tension or encroachment by neighboring hardware. The nerve is transposed as necessary.
TREATMENT OF NONACUTE INJURIES
Hemiarthroplasty may also be used as a salvage reconstruction in cases of malunion, fixation failure, or nonunion after open reduction internal fixation. In these instances, the principles of the reconstruction are the same in terms of restoring the column architecture, preserving the collateral ligaments and recreating the rotation axis (Fig. 52-5). A thorough surgical débridement is essential, and in the case of nonunion, tissue should be sent for histopathology and microbiology assessment to rule out infection. Once tissue is obtained, regional infusion of antibiotics is performed to provide optimal tissue levels in already compromised soft tissues. If supracondylar bone loss is present following débridement, a shortening osteotomy may be required, along with medial and lateral plate fixation. Autologous bone graft is routinely used to promote bony union (Fig. 52-6). The ability to achieve a stable reconstruction should be carefully considered in these complex cases in which bony and soft tissue anatomy may have been significantly altered by prior injury and treatment. If concern exists about this ability then a linked TEA may offer a more reliable reconstructive option. Articular cartilage damage of the radial head and sigmoid fossa due to arthrofibrosis or inflammatory arthropathy may lead to inadequate pain relief, reduced functional outcomes, and uncertain longevity. This represents a relative contraindication that may favor a linked TEA. Thus, if a DHH is planned, it probably should be undertaken early rather than late in a failed ORIF.
PITFALLS AND COMPLICATIONS
Potential pitfalls and corresponding considerations and solutions are listed in Table 52-2. Many of these pitfalls can be addressed by meticulous surgical technique, a thorough understanding of elbow biomechanics, and knowledge of the prosthesis systems as they relate to restoring articular geometry and elbow stability.
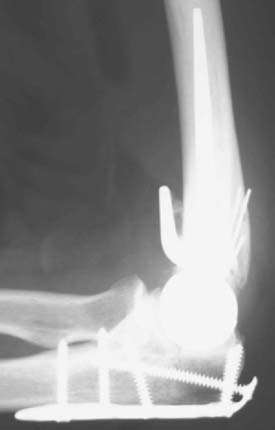
Joint stiffness may occur despite a properly performed reconstruction and early mobilization. If stiffness remains unresponsive to static night splinting, then dynamic splinting can be considered at 2 to 3 months. Persistent stiffness despite these efforts can be managed with contracture release by a column approach at 6 to 12 months postoperatively. Heterotopic ossification, although not common, may occur in the setting of severe elbow trauma (Fig. 52-7A). This is managed by excision and contracture release when the bone is radiographically mature. Instability appears to be related to triceps on exposures, epicondyle comminution, and incorrect collateral ligament reconstruction (see Fig. 52-7B).
RESULTS
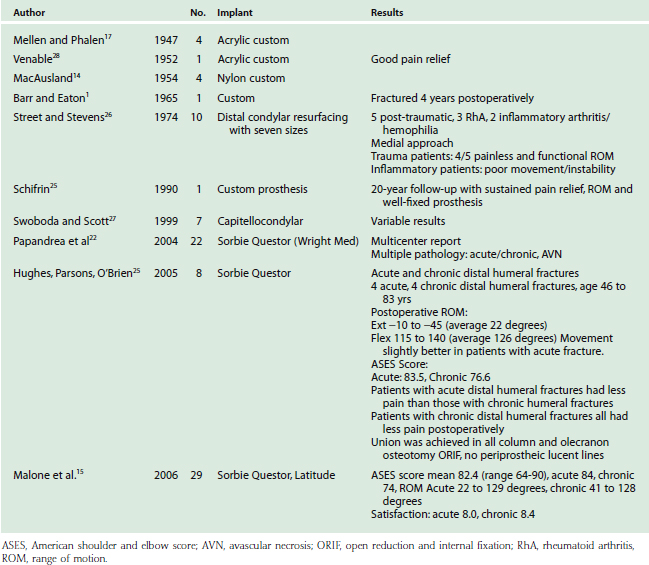
Table 52-3 lists the series of DHH to date. Although long-term prospective data are lacking, except in a few anecdotal case reports, early experience has yielded several observations. First, DHH can lead to substantial pain relief and improvement in function after complex distal humerus fractures. Second, treatment of acute fractures generally results in more favorable outcomes than salvage reconstruction of nonunion, malunion and hardware failure. Third, DHH has proven less effective in chronic conditions such as inflammatory arthritis and hemophilia. Repeat procedures are usually related to hardware removal for fracture or osteotomy fixation. Revision of components is relatively simple especially with the modular systems that can be converted to linked TEA. Future prospective evaluation will better define indications, technique, restrictions and the long-term effectiveness of DHH.
CONCLUSION
Treatment of complex distal humerus fractures or loss of trochlea architecture can be demanding. Although internal fixation and TEA have proven effective in many cases, there are circumstances for which neither represents an ideal treatment. These circumstances include younger or physically active patients. DHH offers an alternative that can provide immediate stability and early mobilization with less risk of implant failure compared with a linked TEA. This is a technically demanding procedure that requires experience with both distal humeral fracture fixation and TEA. Combined techniques may be necessary to restore column architecture and resurface the joint. All supplemental fixation must be rigid enough to allow early motion so that the postoperative recovery from DHH can be reduced to the management of the olecranon osteotomy. Based on early encouraging results, we believe that this is a valuable technique that merits continued refinement through surgical experience and ongoing prospective studies. Our experience indicates a more reliable outcome in the acute (80%) than in the delayed, reconstructed (50%) elbow.16
1 Barr J.S., Eaton R.G. Elbow reconstruction with a new prosthesis to replace the distal end of the humerus: A case report. J. Bone Joint Surg. Am. 1965;47:1408.
2 Beckett K.S., McConnell P., Lagopoulos M., Newman R.J. Variations in the normal anatomy of the collateral ligaments of the human elbow joint. J. Anat. 2000;197:507.
3 Blewitt N., Pooley J. An anatomic study of the axis of elbow movement in the coronal plane: relevance to component alignment in elbow arthroplasty. J. Shoulder Elbow Surg. 1994;3:151.
4 Cobb T.K., Morrey B.F. Total elbow arthroplasty as primary treatment for distal humeral fractures in elderly patients. J. Bone Joint Surg. Am. 1997;79:826.
5 Cohen M.S., Bruno R.J. The collateral ligaments of the elbow: anatomy and clinical correlation. Clin. Orthop. Rel. Res. 2001;383:108.
6 Cohen M.S., Hastings H.N. Rotatory instability of the elbow. The anatomy and role of the lateral stabilizers. J. Bone Joint Surg. Am. 1997;79:225.
7 Faber K.J., Cordy M.E., Milne A.D., Chess D.G., King G.J., Johnson J.A. Advanced cement technique improves fixation in elbow arthroplasty. Clin. Orthop. Rel. Res. 1997;334:150.
8 Floris S., Olsen B.S., Dalstra M., Sojbjerg J.O., Sneppen O. The medial collateral ligament of the elbow joint: anatomy and kinematics. J. Shoulder Elbow Surg. 1998;7:345.
9 Frankle M.A., Herscovici D., DiPasquale T.G., Vasey M.B., Sanders R.W. A comparison of open reduction and internal fixation and primary total elbow arthroplasty in the treatment of intraarticular distal humerus fractures in women older than age 66. J. Orthop. Trauma. 2003;17:473.
10 Gambirasio R., Riand N., Stern R., Hoffmeyer P. Total elbow replacement for complex fractures of the distal humerus. An option for the elderly patient. J. Bone Joint Surg. Br. 2001;83:974.
11 Garcia J.A., Mykula R., Stanley D. Complex fractures of the distal humerus in the elderly. The role of total elbow replacement as primary treatment. J. Bone Joint Surg. Br. 2002;84:812.
12 Gramstad G.D., King G.J., O’Driscoll S.W., Yamaguchi K. Elbow arthroplasty using a convertible implant. Tech. Hand Up. Extrem. Surg. 2005;9:153.
13 Inagaki K., O’Driscoll S.W., Neale P.G., Uchiyama E., Morrey B.F., An K.N. Importance of the radial head component in Sorbie unlinked total elbow arthroplasty. Clin. Orthop. Rel. Res. 2002;400:123.
14 MacAusland W.R. Replacement of the lower end of the humerus with a prosthesis: A report of four cases. Western J. Surg. Gynec. Obstet. 1954;62:557.
15 Malone, A. A., Zarkadas, P. C., Hughes, J., and Jansen, S.: American Shoulder and Elbow Surgeons Open Meeting on Biologics in Shoulder Surgery, November 2006.
16 Malone, A., and Hughes, J.: Hemiarthroplasty for the treatment of distal humeral pathology. (Submitted for publication, 2008).
17 Mellen R.H., Phalen G.S. Arthroplasty of the elbow by replacement of the distal portion of the humerus with an acrylic prosthesis. J. Bone Joint Surg. Am. 1947;29:348.
18 Morrey B.F., An K.N. Stability of the elbow: osseous constraints. J. Shoulder Elbow Surg. 2005;14(suppl S):174S.
19 O’Driscoll S.W., Jaloszynski R., Morrey B.F., An K.N. Origin of the medial ulnar collateral ligament. J. Hand Surg. 1992;17:164.
20 Obremskey W.T., Bhandari M., Dirschl D.R., Shemitsch E. Internal fixation versus arthroplasty of comminuted fractures of the distal humerus. J. Orthop. Trauma. 2003;17:463.
21 Ochi N., Ogura T., Hashizume H., Shigeyama Y., Senda M., Inoue H. Anatomic relation between the medial collateral ligament of the elbow and the humero-ulnar joint axis. J. Shoulder Elbow Surg. 1999;8:6.
22 Papandrea, R., Hughes, J., Sorbie, C., et al: Prosthetic Hemiarthroplasty of the Distal Humerus. ASES Meeting, November 2004.
23 Parsons M., O’Brien R., Jason H., Jeffery S. Elbow hemiarthroplasty for acute and salvage reconstruction of intra-articular distal humerus fractures. Tech Shoulder Elbow Surg. 2005;6:87.
24 Ray P.S., Kakarlapudi K., Rajsekhar C., Bhamra M.S. Total elbow arthroplasty as primary treatment for distal humeral fractures in elderly patients. Injury. 2000;31:687.
25 Shifrin P.G., Johnson D.P. Elbow hemiarthroplasty with 20-year follow-up study. A case report and literature review. Clin. Orthop. Rel. Res. 1990;254:128.
26 Street D.M., Stevens P.S. A humeral replacement prosthesis for the elbow. Results in ten elbows. J. Bone Joint Surg. 1974;56A:1147.
27 Swoboda B., Scott R.D. Humeral hemiarthroplasty of the elbow joint in young patients with rheumatoid arthritis: a report on 7 arthroplasties. J. Arthroplasty. 1999;14:553.
28 Venable C.S. An elbow and an elbow prosthesis. Am. J. Surg. 1952;51:1590.
29 Wevers H.W., Siu D.W., Broekhoven L.H., Sorbie C. Resurfacing elbow prosthesis: shape and sizing of the humeral component. J. Biomed Eng. 1985;7:241.
PART B Radiohumeral Arthrosis: Anconeus Arthroplasty and Capitellar Prosthetic Replacement
ETIOLOGY
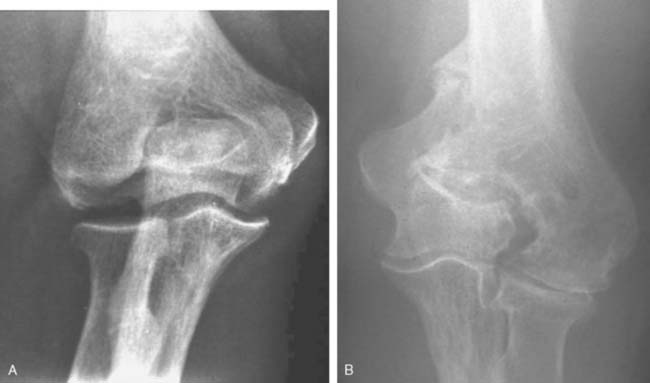
Like so many other pathologic conditions, it seems as though the process is more frequent once we are attuned to investigate for its presence. In general, as a condition, primarily osteoarthritis at the elbow, generally considered uncommon in the past, is today well recognized in the orthopedic community.3,6,10,11 Pathology of the radiohumeral joint occurs from primary osteoarthritis, as a sequelae to osteochondritis dissecans, following capitellar fracture, or secondarily to radial head fracture either ignored, treated by open reduction and internal fixation, or by prosthetic replacement. Finally, the condition is seen after split T and Y type of fractures of the distal humerus resulting in malalignment (Fig. 52-8).
The frequency and impact of these various conditions is not readily available in today’s literature. There has been some interesting studies referable to primary osteoarthritis of the radiohumeral joint that are worth noting. Ortner13 examined the elbow of a population of Eskimos and South American Indians. As a result of this investigation, the authors describe several forms of osteoarthritis at the radiohumeral joint including (1) hypertrophic bone formation peripheral to the articular surface; (2) hypertrophic bone formation in the radial fossa; (3) increased porosity and hypertrophy of the lateral condylar ridge; (4) porosity of the capitellum and; (5) eburnation of the capitellum. These authors note interestingly and accurately that the narrowing of the ulnohumeral joint is not a characteristic of primary osteoarthritis but is more frequently seen at the radiohumeral joint. On the other hand, the osteophyte formation is more commonly observed in the margin of the trochlea and at the coronoid and tip of the olecranon. Subsequently, Goodfellow et al11 documented the increasing incidence of osteoarthritis of the radiohumeral joint with aging and emphasized the early development of the process at the posterior medial ridge separating the trochlea and the capitellum (Fig. 52-9).4 Occupation risks in foundry workers was documented. In general, heavy, repetitive use of the extremity places the joint at risk.
Typically, this process appears to be relatively asymptomatic, but with enhanced awareness and closer scrutiny, the presence of radiohumeral joint symptoms is being increasingly appreciated. These observations have been confirmed somewhat in the recent documentation of intervention for primary osteoarthritis of the elbow. Kelly et al7 described radiohumeral narrowing and instances in which the ulnohumeral joint was débrided arthroscopically. They noted that the radial humeral involvement often typically appears to be well tolerated and need not be directly assessed. Others emphasize the technical ability to débride the ulnohumeral joint but do not comment to any extent on the radiohumeral joint. Although both closed7–9 and open procedures1,14,16 are well-accepted means of treating primary osteoarthritis of the elbow, few make reference specifically to the management of the radial head.7,9 Radiographically, however, the radial head involvement has been documented by Dalal.2 In this study of 50 patients with primary osteoarthritis, radial head involvement was present in approximately 85% but was not typically symptomatic. Some narrowing of the joint was present in approximately 60% compared with only 15% narrowing of the ulnohumeral joint. Savoie’s group is one group that has addressed the radiohumeral component of primary osteoarthritis. These authors perform radial head resection through the arthroscope and, in so doing, document improved motion in both flexion and extension and pronation and supination.9 The long-term impact, however, of removing the radial head in the presence of ulnohumeral joint arthrosis is unknown. This is one of the major issues that prompts one to consider possibly replacing this joint if it is symptomatic enough to deserve treatment on its own merits.
TREATMENT OPTIONS
Experience to date on the treatment of isolated radiohumeral arthrosis or particularly a capitellar arthritis is limited. In general, the treatment options include débridement versus reconstruction. The reconstructive options have relatively little data, but these consist of resecting the capitellum, analogous to isolated resection of the radial head but with little data regarding its outcome. Débridement is effective in those with symptomatic osteochondritis dissecans, particularly when the fragment is loose and causing mechanical symptoms (see Chapters 19 and 38).
Biologic interposition arthroplasty or prosthetic replacement of the capitellum and/or the capitellum and radial head is a consideration. When the capitellum has been destroyed as a result of fracture, often with resorption, a unique problem exists for which there is no good solution. We have performed allograft replacement with or without interposition arthroplasty in a few of these patients. We have also performed an anconeus rotational plasty in patients with deficient or arthritic capitellum and this does seem to be an effective modality. This procedure does not, however, provide axial stability.12 Without question, one of the greatest problems with the radiohumeral joint relates to problems at the articulation after the radial head has been fractured and treated by fixation or prosthetic replacement. Under these circumstances, the radial head is typically excised or the prosthesis is removed. If the medial collateral ligament is stable, this is adequate treatment. If, however, the medial collateral ligament is unstable, then the major indication for consideration of a prosthetic implant exists. This is intended to satisfy the need to stabilize the “lateral column” in instances in which there is deficient medial collateral ligament or axial instability such as an Essex-Lopresti lesion. There is some, but limited, experience with prosthetic replacement to date (Pooley J, personal communication).
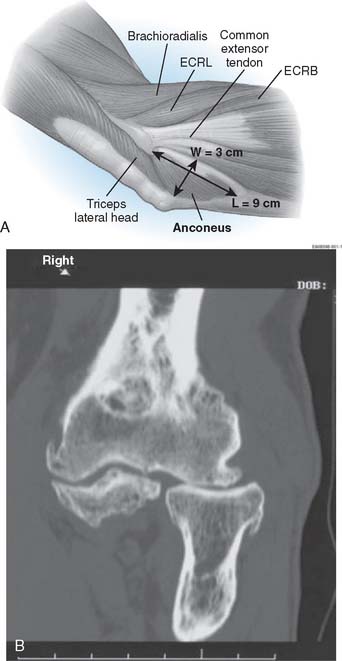
ANCONEUS ARTHROPLASTY
Indications
In general, this technique is employed in instances when the proximal radial ulnar or ulnohumeral dysfunction occurs following trauma or resection, or after primary osteoarthrosis. This procedure was designed for two clinical circumstances: (1) radiohumeral impingement, in which the goal is to buffer the proximal radiocapitellar articulation; and (2) rotatory radioulnar impingement.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree