Uncemented Acetabular Components
Neil P. Sheth and Craig J. Della Valle
Key Points
Introduction
Cemented acetabular component fixation in total hip arthroplasty (THA) was introduced in the early 1960s by Sir John Charnley. Early results demonstrated favorable clinical outcomes with radiographic loosening rates of less than 5%.1 The use of cement remained the major mode of acetabular component fixation until longer-term (>10-year follow-up) results demonstrated rates of radiographic loosening as high as 60%.1–9 Improvements in cement technique throughout the 1970s and 1980s led to improved femoral component durability, but similar benefits were not recognized on the acetabular side.6,10,11 In addition, the surgical technique for cemented acetabular fixation is technically demanding, and even in expert hands, radiographic outcomes were not always found to be ideal. Furthermore, the lack of liner modularity rendered few options for optimizing intraoperative stability. Based on these factors, interest mounted in a new approach to acetabular component fixation. Specifically, biological fixation of acetabular components through insertion of a cementless device developed as an alternative method of reconstruction in the hope that a biological bond between implant and host bone would provide greater long-term durability, along with a simpler, more reproducible surgical technique and greater intraoperative flexibility.
Cementless acetabular fixation brought about the advent of novel designs and cup geometries and the use of different implant materials in conjunction with various surface coatings that would lead to initial implant stability and long-term biological fixation. Factors critical to obtaining adequate stability and biological fixation were identified, and methods of cup insertion were developed. Radiographic and clinical evaluation at more than 20 years postoperatively has shown favorable results12 that have made hemispherical, porous-coated, cementless acetabular devices with or without adjunctive screw fixation the implant of choice in most North American centers for primary total hip arthroplasty.
General Considerations
Requirements for bone ingrowth include biocompatibility of the surface material, an appropriate pore size, adequate surface contact with host bone, and initial stability during the incorporation process.13–16 Pilliar and associates demonstrated that excessive micromotion greater than 150 µm at the bone-implant interface resulted in fibrous tissue infiltration behind the acetabular component as opposed to bone ingrowth.17 Bobyn and colleagues determined that the optimal pore size for bone ingrowth to occur was between 100 µm and 400 µm.18 As a result of these findings, in the early 1980s, porous-coated hemispherical cups were introduced, including porous-coated anatomic (PCA, Howmedica, Rutherford, NJ), Harris-Galante (Zimmer, Warsaw, Ind) (Fig. 66-1), and anatomic medullary locking cups (AML, DePuy, Warsaw, Ind).
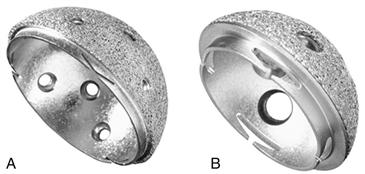
Figure 66-1 Harris-Galante (HG)-1 and HG-2 cups.
Implant Materials
Titanium and Cobalt-Chromium Alloys
Various materials were initially used in the manufacture of cementless acetabular components, including nonmetallic materials (such as all-polyethylene or all-ceramic components, which met with high failure rates as there was no surface by which ingrowth could support long-term stability) and, more commonly, titanium19 or cobalt-chromium alloys. Both metal-based materials are biocompatible and are capable of supporting bone ingrowth; however, quantitatively, titanium has shown increased bone density, deeper penetration, and a greater degree of mean bone ingrowth as compared with cobalt-chromium components.20
Additional benefits are associated with titanium as an implant material. Titanium has a lower modulus of elasticity, closer to that of cancellous bone, theoretically yielding a lesser degree of stress remodeling of the host pelvic bone. In addition, it is a more flexible material, lending itself to easier insertion, especially if a press-fit technique is used, and is associated with potentially lower insertional acetabular fracture risk.21 From a manufacturing perspective, titanium is easier to handle than cobalt-chromium, and, in the arena of health care cost containment, titanium is less expensive.21
Implant Shape
The concept of “cement disease” became popular as a reason for loss of fixation of cemented acetabular implants. By eliminating cement, the hope was that acetabular fixation would be more durable. The first generation of cementless acetabular components were designed with several different geometries and were classified by Morscher and associates22 into five different types: (1) cylindrical, (2) square, (3) cone type, (4) ellipsoid or oblong, and (5) hemispherical. Of these design types, the most popular designs used are presented in the following paragraphs.
Among the more common early “cementless” designs were threaded acetabular components, designed in the early 1970s. Most of these designs did not possess a surface coating and therefore relied upon a mechanical interlock between the device and the host bone, rather than biological fixation, to achieve stable implant fixation. Some specific designs included the Lord cup, along with Mittelmeier, Mecring, T-Tap, and S-ROM Anderson implants. Several studies reported initial satisfactory results, but longer-term follow-up revealed loosening rates of nearly 60% and early revision rates for aseptic loosening as high as 31% at 10 years.23–28
Several theories regarding the reasons for unacceptable rates of migratory failure with first-generation threaded implants became prevalent. It was thought that screwing in a cementless implant led to increased stresses at the implant-bone (thread) interface, resulting in subsequent pressure necrosis, bone resorption, and fibrous tissue interposition.23,29 Retrieval studies supported the postulation that fibrous tissue filled in between the threaded surfaces, yielding inadequate host-bone contact to support long-term fixation.30,31
As compared with hemispherical cup designs, threaded components were more difficult to reproducibly insert in the proper position. Acetabular bed preparation for threaded components was challenging and often led to poorer mechanical stability compared with hemispherical cups because of a lesser degree of surface area contact with host bone. In addition, many systems used an awkward insertional handle, which predisposed to vertical cup positioning, especially in obese patients.21
The failure of first-generation cementless devices brought about so-called second-generation threaded cementless implants that incorporated a porous or grit-blasted surface to enhance biological fixation via bone ingrowth or ongrowth, respectively. Despite the shortcomings of threaded designs, these devices relied upon the threads for initial mechanical stability and the surface coating for long-term biological fixation. Cups with this design included the Zweymüller (grit-blasted titanium; Zimmer), the S-ROM Super Cup (titanium-sintered beads; DePuy), and the Arc-2f (hydroxyapatite-coated; Osteonics, Stryker Orthopaedics, Mahway, NJ) (Fig. 66-2). Medium-term results demonstrated extremely low rates of radiographic failure; however, these devices had numerous problems that resulted in a move toward alternative designs, including difficulty with insertion and high rates of polyethylene wear.28,32,33 Incorporation of a surface coating with the early threaded design feature supported the importance of initial mechanical stability in conjunction with biological fixation for long-term implant stability.
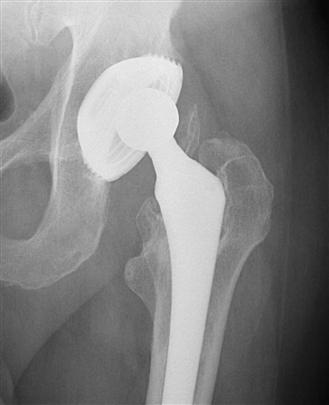
Figure 66-2 Anteroposterior (AP) radiograph of a threaded acetabular cup. (Courtesy Dr. William L. Jaffe, MD.)
Hemispherical cups are classified as having single or dual geometry design. Dual geometry components exhibit an enlarged radius at the rim of the component and were designed to maximize the surface area of contact for biological ingrowth. The dual geometry design gained enthusiasm in attempts to avoid adjunctive fixation to enhance the initial mechanical stability of the implanted acetabular component. Concern has arisen that design features may decrease total contact between the component and the surrounding host bone, specifically at the dome portion of the implant, leading to decreased bone ingrowth. Bauer and coworkers34 evaluated two dual geometry cup designs at the time of postmortem retrieval and found evidence of increased bone ingrowth at the peripheral rim as compared with the dome. The authors compared this design to threaded cups and determined that a greater degree of bone apposition was seen with the dual geometry design, but bone ingrowth was less than was suggested by radiographs.34 Most cementless acetabular devices in use today are single geometry in design, mostly because of the ease of acetabular bed preparation and reproducible insertion.21
The final shape to be considered is the hemi-ellipsoid shape of the Zimmer trabecular metal component. This device exhibits a gradual transition in interference from the peripheral rim (2 mm) to the dome (0 mm). The rationale behind this design is that it maximizes peripheral interference stability, minimizes the potential for early bottoming out, and eliminates the discontinuity associated with dual geometry designs. This interference fit plays an important role in monoblock-type implants, into which screws cannot be inserted for supplemental fixation (Fig. 66-3).
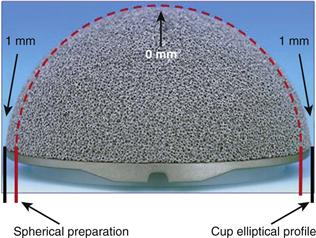
Figure 66-3 Hemi-ellipsoid shape of a trabecular metal acetabular monoblock shell. (Courtesy Zimmer, Warsaw, Ind.)
Surface Coatings
With the understanding that surface coatings were of critical importance in achieving clinical success with an uncemented acetabular component, several different surface preparations were developed. Hydroxyapatite (HA) coatings gained popularity as osteoconductive surface coatings for acetabular components, secondary to good early and intermediate results when applied to cementless femoral components.35,36 Unfortunately, when applied to the back of a cementless acetabular component without an underlying porous surface, when the HA resorbed over time, a high rate of failure ensued, because no ingrowth or ongrowth surface could provide long-term fixation.37 Hydroxyapatite is still popular, however, and improved results have been associated with the use of HA in conjunction with porous-coated or grit-blasted surfaces.38–40
Calcicoat
The basic requirements for bone ingrowth became evident and included factors such as material biocompatibility, optimal pore size, initial implant stability, and intimate contact at the interface between the component and the host bone. Adjuvant techniques, specifically the use of a calcium phosphate ceramic (Calcicoat, Zimmer), were devised to further enhance bony ingrowth into porous materials.41 Ducheyne and associates42 demonstrated increased strength and skeletal fixation and volume of bone formation with the use of porous implants impregnated with a calcium phosphate slurry. Other authors attempted to replicate the study; although they were unsuccessful in reproducing the results,43 the concept of a bioreactive coating on an inert porous metal to enhance biological fixation became attractive.
Rivero and colleagues41 used an in vivo animal model to evaluate porous titanium fiber implants for cementless skeletal fixation treated with a calcium phosphate coating applied with a plasma flame-spray as compared with matched-pair controls. Implants were inserted into the humeri and olecranons of 36 adult dogs and were harvested and biomechanically tested over a 6-week period at 2-week intervals. Calcicoat-treated implants demonstrated 24% greater shear strength (P < .01) than matched-pair controls at 4 weeks. The osteoconductive properties of Calcicoat were thought to allow for bone formation in direct contact with the coating on the titanium fibers. The authors concluded that although the volume of bone growth was not different between groups, Calcicoat as a ceramic coating may serve to enhance the skeletal fixation of porous metal implants.
Other studies have clinically evaluated various coating surfaces such as Calcicoat. Laursen and coworkers44 assessed the 3-year results of porous-coated Trilogy acetabular components as compared with Trilogy acetabular cups coated with HA and tricalcium phosphate (TCP). All cups were inserted using a press-fit technique and were evaluated by dual-emission x-ray absorptiometry (DEXA) scan to determine periprosthetic bone density. At the time of final follow-up, no difference was noted between the two cohorts in terms of periprosthetic bone density. However, the authors concluded that heavier patients regained more bone mineral; this supports the assumption that load is beneficial for bone remodeling.44
Porous Metals
Significant enthusiasm has surrounded the use of recently introduced so-called porous metals that have biomechanical properties similar to those of cancellous bone. Metals such as tantalum possess a volume porosity of 70% to 80%, a modulus of elasticity of 3 GPa (same order of magnitude as cancellous bone), and frictional characteristics that encourage bone ingrowth45 (Fig. 66-4).
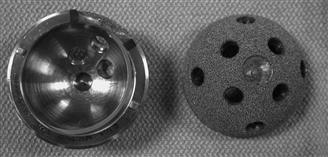
Figure 66-4 Modular tantalum cementless acetabular cup.
Several early clinical and radiographic evaluations were conducted using tantalum acetabular components for primary total hip arthroplasty (THA). These studies described acceptable results comparable with those of conventional cementless components; however, no direct comparative studies suggest a clear advantage over components manufactured from standard materials such as titanium.46–48 In addition, in the single component that has been studied most widely, a high rate of dislocation was observed; this was hypothesized to be secondary to femoral neck impingement against the rim of the polyethylene liner.
In one recent study, Gruen and colleagues48 evaluated 574 patients at 2 to 5 years after the index THA. At final follow-up, only 412 of the original patient cohort were available for radiographic analysis, although 100% demonstrated evidence of osseointegration. A total of 10 hips (1.7%) required revision: six (1%) for recurrent dislocation, one (0.2%) for traumatic cup loosening, and three (0.5%) for infection. Although short-term results seem favorable, longer-term clinical studies are required to determine the efficacy of porous tantalum and other porous metals.
Methods of Uncemented Acetabular Cup Fixation
Immediate implant stability is critical for achieving osseointegration. Although line-to-line insertion and fixation with supplemental screws for fixation were popular in the past, most surgeons currently use a press-fit technique wherein the acetabulum is reamed to 1 to 2 mm below the size of the component inserted with or without the use of supplemental screws, pegs, or spikes for adjunctive fixation.
Supplemental Fixation
Although supplemental fixation with screws has been associated with high rates of osseointegration,31,49 concerns have been raised regarding screws and associated screw holes as pathways for wear particles that lead to osteolysis.50 Further, fretting corrosion between the screws and the metal shell is an additional concern with the use of screws for adjunctive fixation.
Cook and coworkers conducted a histologic study comparing the degree of bone ingrowth attained with the use of components with spikes, pegs, or screws (Fig. 66-5). The authors were able to demonstrate that the distribution of bone ingrowth surrounded the pegs or spikes, but was greater surrounding cups secured with adjunctive screws, because this design allowed visualization of bone-implant contact upon insertion—a factor that has been shown to influence bone ingrowth. They concluded that the use of peripheral spikes or pegs may prevent adequate seating of the cementless device, thereby reducing the surface area of contact between the porous surface and the host bone. Concerns surfaced regarding difficulty in repositioning a malpositioned cup with peripheral fins or spikes. In addition, several retrieval studies have shown increased bone ingrowth adjacent to screws compared with other regions of the cup.31,51

Figure 66-5 Cementless acetabular components with different fixation options, including spikes, pegs, or screws.
Biomechanical analyses of these modes of adjunctive fixation further demonstrated that the use of screws may convert torsional forces to compressive forces, thereby preloading the bone-prosthesis interface and increasing the contact area, further promoting bone ingrowth.52 Using in vitro models, Lachiewicz and associates53 showed that significantly greater torque was required for screw failure as compared with spikes and pegs, and Stiehl and colleagues54 revealed less micromotion with the use of screw fixation compared with pegs.
Although ample literature supports the use of screws, other studies have demonstrated excellent clinical success of components when dome spikes or peripheral pegs and fins were used. Engh and colleagues55 reported 15-year survivorship of 95% for tri-spiked cementless acetabular components with revision for aseptic loosening as an endpoint.
With improved, low wear–bearing surfaces, concerns regarding screw holes as access channels for wear particles may no longer be relevant, because the particle load with alternative bearings and highly cross-linked polyethylene may be below the level required to induce osteolysis. Long-term studies are required to make a definitive conclusion, because this concept pertains to implant longevity.
An additional important consideration to be addressed regarding supplemental screw fixation is the risk of neurovascular injury associated with errant screw placement. Through the combination of defined acetabular quadrants, improved understanding of pelvic anatomy, and increased surgeon experience in placing acetabular screws, the risk for neurovascular injury has been dramatically reduced by placement of screws in the posterosuperior quadrant with careful measurement of screw length.56
Press-Fit Technique
The concept of a pure press-fit technique without the use of screws for component insertion was developed to avoid the use of screws for the reasons previously described. The acetabulum is under-reamed by 1 mm to 2 mm, and then the component is impacted into place. This method of cup insertion is dependent upon the viscoelastic or time-dependent properties of bone to allow deformation and recoil to obtain initial implant stability.52
On initial evaluation of the press-fit technique, it was evident that radiolucencies at the periphery of the acetabular shell were less prevalent; however, gaps at the dome were more common. By 2 years, dome gaps were no longer visible, suggesting bone formation at the pole of the component.57 In vitro analysis, however, showed that gaps greater than 2 mm are not compatible with bone formation and subsequently increase the effective joint space for synovial fluid and wear with debris access to trabecular bone behind the component.58
Both Won and associates and Steihl and colleagues demonstrated in a cadaveric model that press-fit insertion with under-reaming by 1 mm resulted in better component mechanical stability than line-to-line insertion with the use of supplemental screw fixation.54,59 Based on this and other studies, it seems that the depth and accuracy of acetabular reaming may be more important than the presence of supplemental fixation modalities (e.g., spikes, pegs, screws) for attaining mechanical stability.
Finite element models have been used to evaluate loading conditions at the periphery of cementless devices. Acetabular strains registered at the periphery of hemispherical cups inserted using a press-fit technique were the highest, resulting in greater compressive bone forces, thus enhancing cup stability.60,61 Component oversizing of 2 mm rather than 1 mm was needed to augment stability; however, greater amounts of under-reaming increase the risk for acetabular fracture. This may be dependent on acetabular component size; in general, larger amounts of under-reaming are acceptable for larger components.
Comparisons were made between hemispherical and dual geometry cup designs that possess a wider radius at the mouth of the implant compared with the dome; this is intended to increase press-fit at the rim of the acetabulum. Dual geometry designs when tested biomechanically produced higher strain rates at the periphery than were produced by hemispherical cups but resulted in less deformation of the acetabular bone stock. Theoretically, such dual geometry designs would require less force for proper implant seating and would potentially decrease the risk of acetabular fracture upon insertion. However, although higher strains were recorded at the periphery with dual geometry designs, hemispherical cups exhibited better overall implant stability; thus the dual geometry design concept, for the most part, has fallen out of favor.60,61
The risk of creating a periprosthetic acetabular fracture is a concern that surfaced when the press-fit technique of insertion was introduced. This technique of insertion relies upon hoop stresses generated within the acetabulum to help maintain component stability. The tenet that is inherent to this technique is that the host bone is able to tolerate the hoop stresses that are generated. Insertion of significantly oversized components may result in inadequate acetabular component seating and possible fracture of acetabular walls or columns. Kim and associates62 showed that 18 of 30 (60%) acetabular fractures occurred during insertion of cementless devices using a press-fit technique. Acetabular fractures were more likely with components that were oversized by 4 mm than 2 mm. Sharkey and colleagues reported 13 periprosthetic acetabular fractures, of which 9 were recognized intraoperatively, during implantation of a hemispherical cup using a press-fit technique and under reaming by 1 mm to 3 mm. Of the 13 patients, 6 had osteoarthritis, 3 had osteonecrosis of the femoral head, 2 had rheumatoid arthritis, and the remaining patients were treated for developmental dysplasia or a hip fracture nonunion. Fractures identified intraoperatively were addressed by a variety of methods including screw augmentation in and around the cup, use of autologous bone graft at the fracture site, and/or protected weight bearing and immobilization postoperatively. Two cases identified postoperatively required acetabular component revision because of radiographic evidence of component migration. As a result, the authors identified two additional predisposing clinical risk factors for periprosthetic acetabular fracture—osteoporosis and rheumatoid arthritis.63
Polyethylene Liners and Modularity
The introduction of modular polyethylene liners in the 1980s addressed concerns over intraoperative flexibility to maximize stability and allow for modular exchange of the bearing surface in cases of wear. Although this design advancement provided potential benefits, it also introduced unanticipated problems such as backside wear (wear associated with an additional interface between the polyethylene liner and the metal shell) and the potential for liner dissociation.64
Wear of the polyethylene liner seemed to be a greater problem than with cemented, all-polyethylene components. Wear rates exhibited by cementless acetabular cups were in the range of 0.10 mm/yr to 0.25 mm/yr11,50,65-73 compared with 0.07 mm/yr to 0.15 mm/yr2,7,9,10,74 with cemented, all-polyethylene cups. First-generation cementless devices in some instances utilized a liner of inadequate thickness often combined with larger, 32-mm heads that caused increased volumetric wear and wear-related complications. Further, suboptimal locking mechanisms and rough inner surfaces in some cases led to substantial backside wear of the liner, which exacerbated the particle load. Another factor that contributed to increased wear rates was the use of gamma-irradiation in air for polyethylene sterilization during this time.
The effect of liner thickness on polyethylene wear rates has been evaluated by several authors. Contact stresses between the femoral head and the acetabular liner increase as the thickness of the liner decreases. With thin polyethylene liners, the liner behaves like the stiffer underlying metal shell, leading to increased failure rates due to early fatigue failure.32,67 Bartel and associates75 demonstrated via finite element analysis that contact stresses dramatically increased when the liner thickness was less than 6 mm. Berry and colleagues76 reported an increased risk of polyethylene wear and liner fracture when the thickness was less than 5 mm.
Congruency between the liner and the inner surface of the shell is another important component of wear generation in cementless acetabular fixation. Irregularities in the surface from design features such as screw holes or fenestrations may result in areas of unsupported polyethylene, leading to cold flow of the polyethylene and further backside wear. Nonuniform congruency between the two surfaces can result in micromotion between the shell and the liner; this is hypothesized to increase retroacetabular osteolysis.14,50,67,68
Early cementless acetabular components were plagued by poor liner locking mechanisms. The first-generation PCA locking mechanism failed secondary to cracks in the liner and resulting deformation of the antirotation notch in the polyethylene rim.66 The Mallory-Head prosthesis utilized a hexagonal liner locking mechanism, which resulted in excessive motion between the shell and the liner and subsequent increased wear and acetabular lysis.77 Harris-Galante 1 and 2 utilized tines for locking the liner into place. These tines were reported to break, creating the potential for liner dissociation.
In an effort to expand the armamentarium to restore offset and address intraoperative instability in primary THA, different design features such as elevated rims and lateral or extended offset were incorporated into polyethylene liners. One study by Archibeck and coworkers78 evaluated the use of extended offset and its effect on cementless acetabular component fixation. The authors retrospectively reviewed more than 1900 primary THA procedures, among which a 7-mm extended offset polyethylene liner was used in 120 cases. The aseptic acetabular loosening rate was 0.12% for the standard offset liner group compared with 4.0% for the offset liner group at a minimum of 2 years’ follow-up (mean, 3.6 years; range, 2 to 9 years). Acetabular component survivorship was significantly less (P < .01) for the offset liner group compared with the standard liner group at 6 years. The authors concluded that even though offset liners are useful for restoring hip mechanics and may assist in addressing THA instability, the increased loosening rate may be attributed to the transfer of increased torsional loads to the prosthesis.78
It is evident that although benefits are provided by the introduction of modularity in cementless acetabular implants, this approach has been associated with problems. In response to these problems, newer designs have incorporated more secure locking mechanisms often combined with a polished inner cup surface to decrease backside wear. Although newer, more wear-resistant bearing surfaces may decrease the prevalence of some of these issues, the presence of a secure locking mechanism is required for optimal results.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







