Arm pain
Remove from game immediately; if >4 days of arm pain, seek medical attention
Pitch counts
Game
Week
Season
Year
9–10 years old
50
75
1,000
2,000
11–12 years old
75
100
1,000
3,000
13–14 years old
75
125
1,000
3,000
Pitch types
No breaking pitches until bones have matured around puberty (~13 years old)
Multiple appearances
Once removed from the mound, do not return to pitching in the same game
Showcases
De-emphasize and/or avoid, if necessary; give adequate time to prepare with no overthrowing
Multiple leagues
Pitch for only one team at a time, with no overlapping seasons
Year-round baseball
Baseball pitchers should compete in <9 months of baseball each year
Despite the literature supporting the US baseball injury prevention guidelines and the implementation of injury prevention programs, additional research shows that further work must be done to raise public awareness of high-risk throwing activities. A recent study by Ahmad et al. investigated the public perception of UCL reconstruction and found that 31 % of coaches, 28 % of players, and 25 % of parents did not believe that the number of pitches thrown was a risk factor for injury [55]. In addition, 51 % of high school athletes, 37 % of parents, 30 % of coaches, and 26 % of collegiate athletes thought that UCL reconstruction should be performed on players without elbow injury in order to enhance performance. These studies highlight the need for continued endeavors to better educate players, parents, and coaches regarding prevention of overuse throwing injuries [55].
25.7 Valgus Instability/Ulnar Collateral Ligament Injuries
Injury to the UCL was first described in javelin throwers by Waris in 1946 [56]. Since that time, UCL injuries have been reported in increasing frequency among other overhead athletes, particularly baseball pitchers. The UCL is the primary restraint to valgus stress throughout the functional range of motion, between 20° and 120° of flexion, and it is subjected to enormous valgus forces during the throwing motion. These forces approach the ultimate tensile strength of the UCL, and repetition of the overhead throwing motion can lead to attritional injury and/or acute rupture. The most well-studied treatment options for UCL injury include nonoperative management with formal rehabilitation, direct ligament repair, and ligament reconstruction.
Nonoperative management of an isolated UCL injury begins with short-term immobilization to control pain and inflammation, as well as to limit valgus stress on the elbow. This is followed by a comprehensive rehabilitation program as described by Wilk et al., which consists of functional exercises and plyometrics and focuses on pitching mechanics, shoulder kinematics, and motion deficits, as well as strengthening of the core, upper extremities, and lower extremities [57–60]. Once the throwing athlete is pain-free and kinetic chain deficits have been addressed, they may transition to an interval throwing program. This conservative approach is generally indicated in non-throwing athletes and similarly low-demand individuals, and it may also be considered in the immature throwing athlete with a partial tear of the ligament [61]. Skeletally mature, UCL-deficient athletes involved in high-demand throwing sports may not respond well to nonoperative treatment [62, 63]. A study by Rettig reported that 42 % of throwing athletes were able to return to their sport at or above their pre-injury level of play following nonoperative management with appropriate rehabilitation [64]. An injection of platelet-rich plasma (PRP) may be considered, although the data is limited for use in UCL tears. Podesta et al. treated 34 overhead athletes (including 27 professional baseball players) with partial UCL tears with injections of platelet-rich plasma (PRP) and rehabilitation [65]. They reported 88 % excellent results with return to their previous level of competition or higher.
Direct repair of the UCL was initially the treatment of choice for UCL injuries, as early data showed better clinical outcomes when compared to nonoperative treatment [66]. However, additional studies that compared UCL repair to reconstruction found that overhead athletes are more likely to achieve better outcomes and return to their previous level of competition with reconstruction of the ligament [15, 67, 68]. A recent study by Savoie et al. showed that a good indication for UCL repair may be the young athlete with a proximal or distal UCL tear with a good quality ligament. In their retrospective case series of 60 young amateur athletes (mean age, 17.2 years), they reported 93 % good or excellent outcomes following direct repair of proximal or distal UCL tears using suture anchors or suture plication with repair to bone drill holes [69].
Reconstruction of the UCL is often indicated in the high-level overhead throwing athlete who sustains a complete tear of the UCL and wishes to return to throwing sports. Ligament reconstruction is also considered in the throwing athlete who sustains a partial tear of the UCL and continues to have pain and/or instability despite an appropriate course of nonoperative treatment, including a comprehensive rehabilitation program as noted above. Jobe et al. described the first reconstruction technique that afforded players a successful return to competition, utilizing a free-tendon graft placed through bone tunnels in the ulna and medial epicondyle of the humerus in a figure-of-eight fashion [5]. The flexor-pronator origin was detached for the surgical approach, and submuscular transposition of the ulnar nerve was performed. Ten of 16 (63 %) throwing athletes were able to return to their previous level of competition; however, roughly one half of the patients had complications, including five ulnar neuropraxias and one flexor-pronator mass rupture [41].
Since the original figure-of-eight technique was described, multiple modifications have been made in an effort to facilitate anatomic reconstruction, obtain strength similar to the native UCL, and expedite secure graft fixation, all while decreasing morbidity associated with disruption of the flexor-pronator mass and transposition of the ulnar nerve [32, 70–74]. To this end, most modifications have addressed the surgical approach and/or the method of graft fixation on both the ulnar and humeral sides. With regard to the surgical approach, Jobe himself transitioned to a flexor-pronator muscle splitting approach, as described by Smith and Altchek [75], and abandoned obligatory transposition of the ulnar nerve. This modified Jobe technique exhibited improved results with a greater proportion of patients returning to their previous level of play (82 %), as well as a decreased complication rate (12 %) [32].
In 1995, Andrews and Timmerman introduced the American Sports Medicine Institute (ASMI) modification, which utilizes a posterior approach between the two heads of the flexor carpi ulnaris, with elevation of the flexor-pronator mass and obligatory subcutaneous ulnar nerve transposition [67]. This approach leaves the flexor-pronator origin intact and avoids morbidity associated with takedown and repair of the flexor-pronator mass. Cain et al. evaluated the clinical outcome of the ASMI modification in 1,281 throwing athletes and found that 83 % of athletes were able to return to their pre-injury level of competition [76]. Complications occurred in approximately 20 % of the patients, but most (96 %) of these were considered minor, including transient ulnar nerve symptoms.
Modifications for graft fixation have included the docking technique [70], interference screw fixation [71], suture anchor fixation [72], and cortical suspensory fixation [73]. The DANE TJ technique (named in acknowledgement of Drs. David Altchek and Neal ElAttrache, as well as the first professional baseball player to undergo UCL reconstruction and successfully return to competition, Tommy John) is one modification which employs two modern fixation techniques, utilizing interference screw fixation on the ulnar side and the docking technique on the humeral side [74]. To date, the figure-of-eight and docking techniques remain the most well-studied reconstruction techniques with reported long-term outcomes [23, 24, 76–79]. However, regardless of the fixation used, most modern techniques have similar outcomes, with 80–90 % of athletes returning to their previous level of play. An overall complication rate has been reported of between 15 and 20 %, with most consisting of transient ulnar neuropathy and superficial wound infection at either the graft harvest site or the elbow [23, 24, 76–79].
Following surgery, the patient should engage in a four-phase rehabilitation program as described by Wilk et al. and noted above [57–60]. The first phase begins immediately after surgery and continues for 3 weeks. Following the UCL reconstruction, the patient’s arm is placed in a posterior splint to immobilize the elbow at 90° of flexion. The splint is kept in place for 1 week to allow for initial wound healing, and the patient is permitted to perform wrist and hand range of motion and hand grasping exercises during this time. After 1 week, a hinged brace is applied and adjusted to allow motion from 30° to 100° of elbow flexion. The elbow motion is increased in a stepwise fashion until the patient achieves full range of motion by the end of the fifth to sixth week after surgery. The hinged elbow brace is discontinued at the end of the 8th week. During phase II (weeks 4–10) and phase III (weeks 10–16), the patient works on progressive strengthening and continued stretching and flexibility exercises. By week 12 the patient is permitted to begin an isotonic lifting program, including bench press, latissimus dorsi pull downs, seated rows, triceps push downs, and biceps curls. Week 12 also marks the time when the throwing athlete may begin a plyometric throwing program. The first 2 weeks of the plyometric program consist of two-hand throws, such as chest passes, soccer throws, and side throws. During the following 2 weeks, the patient is allowed to transition to one-hand throws. Phase IV (weeks 16 and beyond), the return to activity phase, consists of a formal interval throwing program. Throwing athletes are permitted to begin throwing from the mound approximately 6–8 weeks after initiation of the interval throwing program, and return to competitive throwing can be expected 9–12 months after surgery [57–60].
25.8 Ulnar Neuritis
Ulnar nerve neuritis can also occur in overhead throwing athletes secondary to the nerve’s position at the medial elbow, where it is susceptible to compression and traction as well as to inflammation of nearby stabilizing structures. On presentation, athletes typically complain of pain at the medial elbow and sensory disturbance in the ulnar aspect of the hand as well as the ring and small fingers. Overt motor weakness is rare in the thrower, but it can instead present as loss of ball control or difficulty with performance of complex hand tasks. On exam, the physician should determine if there is subluxation or dislocation of the nerve with palpation or elbow range of motion (Fig. 25.1). Patients may also exhibit a positive Tinel sign at the cubital tunnel, as well as a positive elbow flexion test, which reproduces pain, numbness, and tingling in the ulnar nerve distribution with maintained maximum elbow flexion and wrist extension for at least 1 min [80].
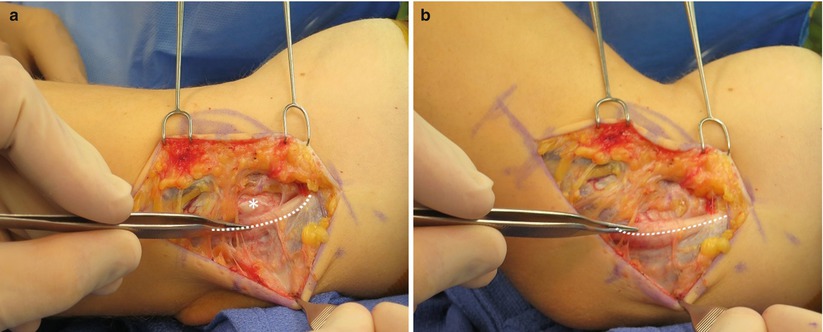

Fig. 25.1
(a) Intraoperative examination of the ulnar nerve (dashed line) with the arm in extension identifies the nerve located in its anatomic position behind the medial epicondyle (*). (b) In the setting of ulnar nerve instability, flexion of the arm at the elbow results in dislocation of the nerve (dashed line) anterior to the medial epicondyle (Copyright Daryl C. Osbahr)
In addition to standard radiographic imaging, electrodiagnostic studies including electromyography (EMG) and nerve conduction velocities (NCV) may be obtained as part of the diagnostic work-up in cases with equivocal findings on the physical examination. However, results of such studies must be interpreted with caution, as negative test results do not rule out the diagnosis of ulnar neuritis and symptoms of dynamic compression or traction. Rather, positive findings are typically seen only with chronic or advanced nerve entrapment [18, 26, 33]. Symptoms of ulnar nerve inflammation or compression should alert the physician to possible underlying elbow instability. In a systematic review of athletes undergoing UCL reconstruction, approximately 30 % endorsed concomitant ulnar neuropathy [81]. Similarly, ulnar nerve symptoms are reported in as many as 60 % of throwing athletes with medial epicondylitis. Treatment options for ulnar neuritis include nonoperative management, decompression, medial epicondylectomy, and anterior submuscular or subcutaneous transposition.
Treatment of isolated ulnar neuritis should begin with nonoperative management, including cessation of sports activities, rest, ice, and nonsteroidal anti-inflammatory drugs (NSAIDs). In the presence of nerve subluxation or dislocation, a 2-week trial of immobilization may be indicated, as well. Once the patient is asymptomatic, a stretching routine may be established for the elbow, forearm, and wrist, followed by a progressive isometric strengthening program and gradual return to sport-specific functions [33]. Greater duration and severity of symptoms, as well as presence of concomitant valgus instability, may predict decreased success with nonoperative treatment [82, 83].
Surgical intervention may be considered when nonoperative management fails or when the patient presents with advanced symptoms, such as motor weakness or muscular wasting. There is limited data on use of simple decompression or medial epicondylectomy to treat ulnar neuritis in throwing athletes. However, in the throwing athlete, decompression alone is generally not recommended, as it does not eliminate traction force on the ulnar nerve, and medial epicondylectomy may destabilize the UCL or FPM as well as predispose to ulnar nerve subluxation or dislocation [37, 83, 84]. Most of the available literature focuses on anterior submuscular or subcutaneous transposition of the nerve. Historically, some authors have recommended submuscular transposition for the potential advantage of better protection of the ulnar nerve from direct and indirect trauma [17, 18, 20, 33, 34, 41]. More recently, there has been increasing support for subcutaneous transposition in throwing athletes, as this avoids morbidity associated with disruption of the flexor-pronator mass, especially in overhead athletes [23, 67, 68, 85, 86].
Regardless of the method of surgically addressing the ulnar nerve, the nerve must be adequately released and mobilized to ensure that there is no tethering or compression of the nerve along its entire course. Particular attention should be made to free the nerve proximally from the arcade of Struthers and distally from the fascia between the two heads of the FCU, as these areas have been identified as common causes of incomplete release and recurrent ulnar nerve symptoms [87–89].
We prefer subcutaneous transposition for the aforementioned reasons. The surgical approach is similar to that used for UCL reconstruction, and it begins with a 4–5 cm incision centered over the medial epicondyle. The medial antebrachial cutaneous nerve is identified and protected, and the ulnar nerve is released from the cubital tunnel, as well as its proximal and distal restraints, as noted above. A fascial sling is created from a strip of the medial intermuscular septum. The ulnar nerve is transposed anterior to the medial epicondyle, and the fascial sling is laid loosely over the nerve and sutured to the fascia of the FPM. The elbow is then taken through a gentle range of motion to ensure that the ulnar nerve is able to move freely without compression or tethering. The cubital tunnel and fascia of the FCU are both closed. Meticulous hemostasis is obtained using electrocautery, and a drain is placed with plans for removal before discharge home the same day. The wound is closed in two layers, including a subcuticular closure reinforced with Steri-Strips (3 M, St. Paul, Minnesota). The elbow is splinted at 90° of flexion for 1 week. Following splint removal, the patient is permitted to begin progressive range of motion exercises and rehabilitation.
25.9 Flexor-Pronator Injuries
The flexor-pronator musculature provides dynamic stability to the medial elbow, and it assists the UCL in creating the varus torque necessary to counteract the valgus forces created during the overhead throwing motion. Repetition of the throwing motion can lead to muscular fatigue, chronic tendinosis, attritional injury, and acute rupture of the FPM [12, 90]. On presentation, the throwing athlete will typically describe pain during the late cocking and acceleration phases of the throwing motion. Examination usually reveals tenderness just distal to the FPM origin on the medial epicondyle, and resisted wrist flexion and forearm pronation exacerbate pain. The most significant differential diagnosis which must be ruled out is concomitant injury to the UCL. Studies have shown that FPM injuries accompany approximately 4.3 % of UCL injuries, and the risk of combined FPM and UCL injuries among baseball players increases after 30 years of age [15, 91].
The vast majority of FPM injuries respond well to conservative treatment, including rest, ice, and a course of anti-inflammatory medication, followed by physical therapy and a gradual return to throwing. Surgery is considered in throwers with chronic tendinosis, which does not respond to at least 3–6 months of nonoperative treatment, and in the rare case of complete rupture with associated valgus instability.
The literature on operative treatment and outcomes of FPM injuries is limited. A study by Vangsness showed that approximately 90 % of patients with isolated chronic tendinosis have a good or excellent result, and >95 % of athletes are able to return to sports activities, following detachment of the FPM origin, excision of abnormal tissue, and reattachment of FPM [92]. A more recent case series by Osbahr et al. identified a population of baseball players undergoing UCL reconstruction who sustained concomitant flexor-pronator injuries [91] (Fig. 25.2). Compared to baseball players with isolated UCL injuries, baseball players with combined flexor-pronator and UCL injuries were found to be significantly older (33.4 years versus 20.1 years) and had a significantly lower rate of return to prior level of play (12.5 %) [91].
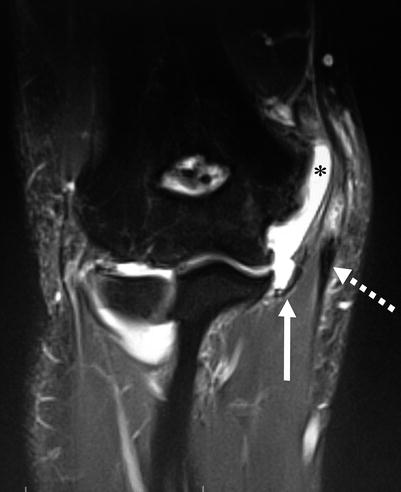

Fig. 25.2
Coronal T2-weighted MR arthrogram of the elbow demonstrating combined injury to the ulnar collateral ligament (solid arrow) and flexor-pronator mass (dashed arrow). Note the abnormal proximal extension of intra-articular contrast (*) to the level of the medial epicondyle (Copyright Daryl C. Osbahr)
25.10 Medial Epicondyle Apophyseal Injuries
Recent decades have seen an increase in elbow injuries in youth baseball pitchers [53]. This has been attributed to high pitch counts and increased sport participation, including year-round league play, involvement in concurrent leagues, and travel team play [51, 52]. While adolescent athletes are susceptible to UCL injuries, the substantial valgus forces created by the overhead throw more typically affect the relatively weak medial epicondyle apophyseal plate, resulting in medial epicondyle apophysitis and avulsion injuries [12].
Classically, medial epicondyle apophyseal injuries have been thought to result from repetitive microtrauma over a prolonged time period. Early studies by Bennett and Brogdon introduced the concept of “Little Leaguer’s elbow” to describe the clinical and radiographic findings discovered in the throwing arms of youth baseball players [93, 94]. Their patients were noted to present with a prior history of pain, swelling, and tenderness at the medial elbow, and radiographs revealed fragmentation and physeal widening at the medial epicondyle. More recent studies have corroborated their initial reports; however, the aforementioned chronic radiographic findings have since been seen among asymptomatic adolescent baseball athletes, including both pitchers and position players [95–100]. This has created some controversy regarding the overall significance of these findings, as well as their exact incidence among asymptomatic youth throwers. Depending on the study population, the incidence of radiographic widening and fragmentation of the medial epicondyle has been reported to range from 4 to 50 % [95, 97–100].
While medial epicondyle apophysitis generally presents with chronic complaints and findings, medial epicondyle avulsion fractures may occur with a characteristic acute presentation while throwing. A case series by Osbahr et al. reported on eight previously asymptomatic youth baseball players who experienced a sudden acute avulsion fracture during the act of throwing [95]. Patients typically reported a sudden pain or “pop” while throwing and presented with acute pain, swelling, and tenderness, as well as decreased range of motion [95]. Plain radiographs are usually sufficient for diagnosis and most often reveal a Salter-Harris type I fracture, but fragmentation of the epicondyle may be observed. A CT scan may be considered to determine total fracture displacement, as well as to assist with treatment decision making.
As previously noted, prevention is key in the management of elbow injuries in youth athletes, and adherence to the USA Baseball Medical & Safety Advisory Committee guidelines has been shown to correlate with the incidence of youth pitching-related arm pain and pitching-related injuries [48–54]. Beyond prevention, the management of medial epicondyle apophysitis is generally straightforward, and good results have been achieved with rest, ice, and activity modification with occasional bracing or splinting [97]. However, there is still much debate within the literature about the optimal treatment of medial epicondyle fractures [12, 91, 101–104]. Many authors agree that non-displaced fractures may be adequately treated with a brief period of immobilization in a long-arm splint or cast with the elbow flexed to 90°, yet there [12, 103]. Considerable controversy regarding treatment of minimally displaced (2–5 mm) medial epicondyle fractures in throwing athletes, as based upon the notion that minimal degrees of valgus instability may be less tolerable in this population. Surgical decision making is further complicated by the fact that the magnitude of fracture displacement may be underestimated by standard radiographs, and the fact that there is low interobserver and intraobserver agreement as based upon standard radiograph measurements [105, 106]. A CT scan may be obtained if there is uncertainty regarding fracture displacement and optimal treatment, but it comes with the risk of increased radiation exposure. Absolute surgical indications typically include open fractures, gross elbow instability, incarceration of the fracture fragment, or entrapment of the ulnar nerve [103, 107].
When operative treatment is indicated, most authors support open reduction and internal fixation with a single screw, with or without a washer [95, 103, 104]. Postoperatively, the elbow is immobilized at 70–90° of flexion with the forearm in neutral rotation for a maximum of 3 weeks. Patients are then placed in a hinged elbow brace to resist valgus forces. Rehabilitation begins at 3 weeks with physical and occupational therapy to work on range of motion, followed by progressive strengthening and gradual return to physical activity. A throwing program may begin once there is radiographic evidence of fracture union, good upper extremity strength, and pain-free range of motion [95].
25.11 Osteochondritis Dissecans of the Capitellum
Osteochondritis dissecans of the capitellum is another condition that is seen primarily in the adolescent overhead athlete. The exact etiology of this disorder remains controversial, but it is believed to be multifactorial and strongly associated with repeated microtrauma to the poorly vascularized immature capitellum [21, 108, 109]. Vascular studies have shown that the capitellum is primarily supplied by posterior end arteries that traverse the articular cartilage, and there is an absence of significant metaphyseal collateral blood flow [21–111]. The overhead throwing motion produces significant compression forces in the lateral compartment of the elbow, which are believed to cause injury to the aforementioned subchondral end arteries, resulting in ischemia, osteonecrosis, and formation of loose bodies [12, 21, 109, 112].
Historically, the management of capitellar OCD has been based upon multiple factors, including the grade and size of the lesion, as well as the state of the capitellar physis [108, 109, 112–114]. Multiple grading systems have been established and are based upon the appearance of the OCD lesion on plain radiographs, CT, MRI, and arthroscopy [112, 115–117]. In general, each of these systems grades the lesion as stable, unstable but attached, or detached and loose. Nonoperative management is typically reserved for patients with stable lesions and an open capitellar physis, and it includes activity modification, use of nonsteroidal anti-inflammatory drugs (NSAIDs), and cessation of sports participation for 3–6 months. Recent data has shown that approximately 90 % of such patients can expect spontaneous healing with nonoperative management [112, 113].
Operative management is indicated in patients with stable lesions that have failed 6 months of nonoperative management and in patients who present with unstable lesions, articular loose bodies, or mechanical symptoms. The goals of surgery are stimulation of a healing response, removal of loose bodies, and resolution of mechanical symptoms. Surgical treatment options include arthroscopic versus open removal of loose bodies, capitellum debridement, abrasion chondroplasty, fragment excision, fragment fixation, microfracture, humeral osteotomy, or osteochondral autograft transplantation surgery (OATS procedure). Most of the outcome data for surgical treatment of capitellar OCD comes from retrospective case series, which had small sample sizes and/or did not utilize modern arthroscopic techniques [118–126]. This makes it difficult to draw significant conclusions or recommend one procedure over another. A systematic review by de Graaff et al. reported on the findings of nine such studies with 219 total patients undergoing arthroscopic treatment for OCD of the capitellum [127]. This included 41 patients who underwent osteochondral autografting and 178 patients who underwent debridement, drilling, microfracture, and/or fragment fixation depending on the grade of their osteochondral lesion. Those patients undergoing osteochondral autografting had a return to sport rate ranging from 77–90 %, and 94 % were pain-free, while all other patients had a return to sport ranging from 80–100 %, and 84–100 % were pain-free [127].
Ruchelsman et al. provided a useful algorithm on capitellar OCD treatment [21]. They recommend retrograde drilling for lesions with intact overlying cartilage and arthroscopic debridement with marrow stimulation (microfracture) for lesions with unstable cartilage caps or loose bodies. Open osteochondral autograft and allograft procedures are reserved for large defects that involve more than 50 % of the width of the articular surface or that engage the radial head [21]. When an open approach is desired, either a direct lateral or posterolateral approach to the elbow may be used, depending on the location of the lesion.
25.12 Posteromedial Impingement
Impingement of the posteromedial bony and soft tissue structures may occur with the repetitive elbow extension and valgus forces created by the overhead throwing motion, particularly in the setting of UCL insufficiency. Such impingement can result in soft tissue swelling, osteophyte formation, chondromalacia, and the development of intra-articular loose bodies. Athletes may complain of pain at the posterior elbow, swelling, crepitus, locking, and/or loss of terminal extension, and they are likely to present with a positive valgus extension overload test [14, 128–130]. Plain radiographs, especially axial and oblique views, can help identify posterior elbow osteophytic changes, and MRI with intra-articular contrast can be performed to detect loose bodies and inflammation of soft tissue structures.
Treatment of posteromedial impingement begins with prevention, including the early recognition and prompt treatment of UCL insufficiency. Nonoperative management typically consists of NSAIDs and active rest, followed by rehabilitation focusing on the entire kinetic chain, including the lower extremities, core, scapular shoulder, and elbow. Elbow rehabilitation should focus on range of motion, flexibility, and flexor-pronator strengthening. As symptoms resolve, the athlete may be permitted to begin a throwing mechanics program followed by a progressive interval throwing program and a gradual return to competition. If the patient does not obtain relief despite adequate rehabilitation, they may be considered a candidate for arthroscopic or open debridement of the elbow with focus on osteophyte excision, treatment of chondromalacia, and removal of loose bodies.
Arthroscopic debridement has become the treatment of choice, as it allows excision of loose bodies, direct visualization of articular surfaces, drilling of osteochondral defects, and evaluation of the UCL for undersurface tears (Fig. 25.3). To date, there have been very few studies which have specifically investigated the outcomes of arthroscopic debridement for the treatment of posteromedial impingement. Rahusen et al. reported on 16 athletes with isolated posterior impingement who underwent arthroscopic debridement of the olecranon and posterior fossa [131]. There was no comparison group, but their cohort had statistically significant improvement in the modified Andrews elbow scoring system (69/100 preoperatively versus 93/100 postoperatively) and the visual analog scale for pain, both at rest (3/10 versus 0/10) and with activity (7/10 versus 2/10) [131]. Outcome data from other studies has shown that arthroscopic treatment with debridement, olecranon osteophyte excision, and loose body removal has permitted 72–85 % athletes to return to play at their previous level of competition [31, 132, 133]. Additionally, the American Sports Medicine Institute’s 2-year follow-up data on UCL reconstruction showed that athletes had an equivalent or higher return to play rate (86 % versus 82 %) when olecranon osteophyte excision was performed at the same time as their UCL reconstruction, compared to performing UCL reconstruction alone [76]. In the same study, arthroscopic debridement of an olecranon osteophyte was the most common reason for additional surgery, and reoperation for olecranon osteophyte excision after UCL reconstruction carried a worse prognosis for return to play at the same level of competition or higher (71 %) [76].
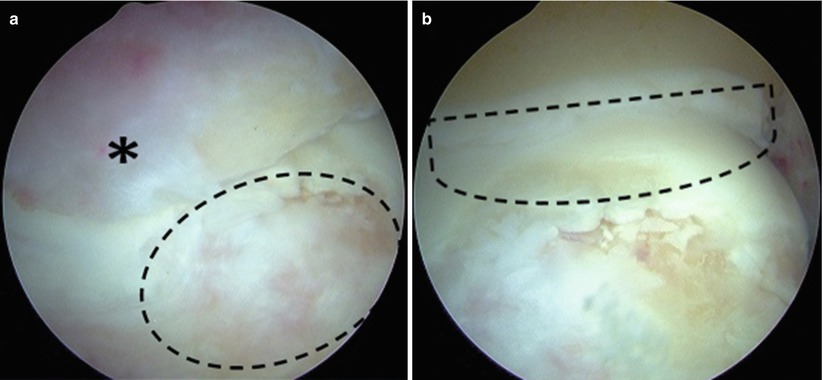

Fig. 25.3




(a) Arthroscopic examination of an athlete with posteromedial impingement reveals a large posteromedial olecranon osteophyte (*) and associated chondromalacia of the humeral trochlea (circle). (b) Removal of the olecranon osteophyte (dashed line) reveals more extensive cartilage damage and allows further evaluation and treatment of the posterior humeral trochlea (Copyright Daryl C. Osbahr)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






