1. Precise and controlled release of the capsule
2. Prevention of fracture and rotator cuff injury
3. Releases and capsulotomies performed with an RF* device
(a) Reduces intra-articular bleeding, preventing further adhesions
(b) Delays capsular healing allowing an end range of motion rehabilitation program
20.2 Surgical Anatomy and Preoperative Planning
There are four anatomic layers at the anterior shoulder girdle. The capsule with the glenohumeral ligaments and the subscapularis tendon constitute the first and the second layers. The coracohumeral ligament (CHL) forms the third layer, while the coracoid along with the coracoacromial ligament forms the fourth layer.
During the symptomatic phases of frozen shoulder, all layers act in concert to trigger symptomatology, whereas CHL appears to be primarily responsible for the severe decline of range of motion. CHL arises from the coracoid base and ends at the upper part of the bicipital groove (Fig. 20.1). This ligament constitutes the main target for surgical capsular release [13, 14].
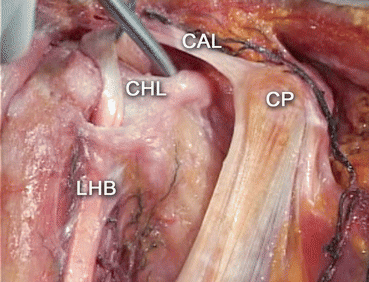

Fig. 20.1
Right shoulder. Anatomic dissection. Rotator interval anatomy. CP coracoid process, CHL coracohumeral ligament, CAL coracoacromial ligament, LHB long head of the biceps (Courtesy of Pau Golano, MD. Barcelona, Spain. With permission)
There is considerable debate regarding the optimal amount of surgical release during ACR. Along with CHL and rotator interval release, some authors advocate releasing other structures including the subscapularis tendon, the inferior and posterior capsule, and the global capsule [15–17] to improve shoulder elevation, internal and external rotations. Pearsall et al. [18] reported that preoperative assessment of motion loss should guide the degree of capsular release.
Although the best timing to proceed with ACR is still controversial, failure of conservative measures and the patients’ wishes for a quicker recovery should be taken into consideration during surgical decision-making. We usually defer surgical treatment of concomitant shoulder injuries such as cuff repair or biceps tenodesis until frozen shoulder recovery. Table 20.2 details the main indications for surgical release. Table 20.3 lists evidence-based data about current treatment modalities for frozen shoulder.
Table 20.2
ISAKOS UECa consent indications for ACR
1. Failure of a well-performed rehabilitation program for a 6-month interval |
2. Failureb after at least 3 steroid injections in a 6-month interval |
3. Failure of less invasive treatments like joint distension or MUA |
4. Severe frozen shoulder in patients with diabetes mellitus in whom steroid treatment is contraindicated |
5. Patient wishes for a faster recovery |
Table 20.3
Clinical evidence regarding treatment
Year | Study size | Treatment comparison | Study design | Outcome | Time | Findings |
|---|---|---|---|---|---|---|
2006 [19] | 100 | High-grade mobilization technique (n = 49) vs. low-grade mobilization technique (n = 51) | RCT | VAP, SDQ, shoulder rating questionnaire, SF-36 | 12 M | High superior to low-grade mobilization rehab techniques |
2007 [20] | 36 | MUA (n = 16) vs. hydrodilation (n = 20) | RCT, single center | VAP, constant score, ROM | 6 M | Hydrodilation superior to MUA |
2007 [8] | 28 | End range motion (ERM) and mobilization with movement (MWM) vs. mid-range of motion (MRM) | RCT | Flex SF and ROM | 3 M | ERM and MWM better than MRM |
2007 [21] | 125 | MUA (n = 16) and home exercises (n = 65) vs. home exercises alone (n = 60) | RCT, single center | SDQ, measures of active and passive ROM | 12 M | Equivalent |
2008 [22] | 76 | Intra-articular shoulder injections with (n = 39) vs. without hydrodilation (n = 37) | RCT, single center | SPADI, measures of active and passive ROM | 1.5 M | Equivalent |
2009 [5] | 53 | Intra-articular shoulder injections using steroid with distension (n = 25) vs. MUA (n = 28) | RCT, single center | Constant score, VAP, SF36 questionnaire | 24 M | Equivalent |
2010 [23] | 74 | ACR with (n = 32) vs. without (n = 42) release of inferior and posterior structures | RCT, single center, single-blind | ROM and VAP | 28 M | Equivalent |
2012 [9] | 44 | MUA and arthroscopic release (n + 23) vs. intra-articular steroid injection (n = 21) | RCT, multicenter | Constant and Murley, ASES, UCLA and SST evaluation scales | 12 M | Equivalent but arthroscopic release leads to faster recovery |
2012 [24] | 45* | Intra-articular steroid injection plus home stretching exercises (n = 23) vs. home stretching exercises alone (n = 22) | RCT | VAP, ASES | 2 M | Steroid injections lead to better outcomes in patients with diabetes |
2012 [25] | 70 | Intra-articular hyaluronic acid injections plus physical therapy (n = 35) vs. physical therapy alone (n = 35) | RCT, single center | SDQ, SPADI, measures of active and passive ROM, SF-36 | 3 M | Equivalent |
2013 [26] | 191 | Four groups. Steroid injection at subacromial space (Group I, n = 49), at glenohumeral joint (Group II, n = 48) Injections at both subacromial space and glenohumeral joint (Group III, n = 47) or NSAIDS treatment (Group IV, n = 49) | RCT, single center | VAP, measures of active and passive ROM, SDQ | 3 M | Glenohumeral vs. subacromial steroid injections were equivalent and steroid injections better than NSAIDs |
2013 [27] | 53 | High vs. low dose steroid injection vs. lidocaine injection | RCT, triple-blind | VAP, SPADI | 3 M | Steroid injection superior to control injection. Equivalent between steroid high and low dose |
2013 [28] | 68 | High (1:30,000, n = 22) and low dose (1:10,000, =23) bee venom acupuncture vs. sham acupuncture (n = 17) | RCT, double-blind | VAP, SPADI, measures of active and passive ROM | 3 M
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|





