Abstract
Introduction
Chronic obstructive pulmonary disease (CPOD) is a severe, incapacitating pathology. Inspiratory and/or expiratory muscle training may favorably impact the indicators of both specific and general improvement with regard to this disease. We are hypothesizing that when combined with bronchial decluttering, this training will have a beneficial effect on lung function and quality of life in these patients.
Method
Fourty COPD subjects classified Gold I and Gold II and aged 60.38 ± 8.02 years were divided into four groups of 10. Three of the groups were trained with the help of Threshold ® tools used for (1) inspiratory, (2) expiratory and (3) inspiratory and expiratory purposes; their training supplemented the decluttering and lower limb muscle exercise that the 4th group concurrently received. The patients underwent 16 rehabilitation sessions over an 8-week period. The variables consisted in: (1) forced expiratory volume in 1 s (FEV1) and spirometrically measured peak expiratory and inspiratory flow rates (PEFR and PIFR); (2) fatigability, dyspnea, heart rate and walking distance evaluated during the 6-minute walk test; (3) maximum inspiratory pressure and (4) maximum expiratory pressure as assessed by the Threshold ® tools and (5) the signs of quality of life in terms of the Saint-George’s respiratory questionnaire (SGRQ) score.
Results
Only in group 1, there was significant improvement with regard to FEV1 and PEFR. There was no PIFR modification in any of the groups. On the other hand, signs of quality of life scores along with dyspnea, fatigability and heart rate showed significant improvement in the three experimental groups, and significant improvement in maximum inspiratory pressure was observed in groups 1 and 3.
Discussion
When associated with decluttering techniques, diaphragmatic rehabilitation and lower limb muscle exercise along with psychological support and educational efforts, respiratory muscle training is beneficial when compared with the usual protocols in rehabilitation of COPD patients.
Résumé
Introduction
La broncho-pneumopathie chronique obstructive (BPCO) est une maladie incapacitante. L’entraînement des muscles inspiratoires et/ou expiratoires pourrait avoir des effets bénéfiques sur les indicateurs d’amélioration spécifique et générale de cette maladie. Nous supposons que cet entraînement, associé au désencombrement, aura un effet bénéfique supplémentaire sur la fonction pulmonaire et la qualité de vie de ces patients.
Méthode
Quarante sujets BPCO (classés grades I et II selon Gold), âgés de 60,38 ± 8,02 ans, ont été divisés en quatre groupes de dix sujets chacun. Trois groupes ont été entraînés à l’aide des outils Threshold ® selon trois modalités en plus des techniques de désencombrement et de l’entraînement des muscles des membres inférieurs que le quatrième groupe a reçu : (1) inspiratoires, (2) expiratoires, (3) inspiratoires et expiratoires. Les patients ont bénéficiés de 16 séances de rééducation sur huit semaines. Les variables ont été : (1) le volume expiratoire maximal à la première seconde (VEMS), les débits expiratoire et inspiratoires de pointe (DEP et le DIP), (2) la fatigabilité, la dyspnée, la fréquence cardiaque et la distance de marche évaluée lors du test de six minutes de marche, (3) la pression inspiratoire maximale, (4) et la pression expiratoire maximale évaluée par les outils Threshold ® , et (5) les signes de la qualité de vie évalués par le score du questionnaire de Saint-Georges (QSG).
Résultats
Il y a une amélioration significative de la mesure du VEMS et du DEP du groupe 1 uniquement. Les DIP de tous les groupes n’ont pas été modifiés. Les signes de la qualité de vie, la dyspnée, la fatigabilité et la fréquence cardiaque ont présenté des améliorations significatives pour les trois groupes. Une amélioration significative de la pression inspiratoire maximale est notée chez les groupes 1 et 3.
Discussion
L’entraînement des muscles respiratoires associé aux techniques de désencombrement, de rééducation diaphragmatique, de l’entraînement des muscles des MI, du soutien psychologique et de l’éducation des patients se révèlent bénéfique dans la réadaptation des patients BPCO relativement aux protocoles habituels.
1
English version
1.1
Introduction
Chronic obstructive pulmonary disease (CPOD) affects persons over 40 years of age who are smokers or exposed to smoke. Following myocardial infarction, strokes, community-acquired respiratory infections and tuberculosis, it represents the 5th cause of death in the world. The World Health Organization (WHO) has hypothesized that by 2020, CPOD mortality shall have doubled with regard to 1990 on account of increased smoking, its main cause, particularly in women .
Initially and lengthily virtually asymptomatic, CPOD is first manifested by coughing and expectoration in the morning, which are followed by dyspnea on exertion and subsequently at rest, and it may finally limit daily activities and constitute a sizable handicap prior to becoming life-threatening.
This disease necessitates targeted prevention, early diagnosis and appropriate care built essentially around respiratory rehabilitation. The results of a number of meta-analyses confirm with a high level of evidence that when applied to CPOD subjects, rehabilitation (bronchial decluttering, reeducation and exercise of the lower limb muscles) engenders improvement in terms of dyspnea, exercise tolerance and quality of life. By diminishing the frequency and duration of hospitalization it reduces the costs associated with the latter.
Three main criteria help to assess functional capacity deterioration in the CPOD patient: lower forced expiratory volume in 1 second (FEV1), diminished quality of life, and the rate of mortality . Indeed, evolution of the disease leads to a severe decline in quality of life .
1.1.1
The inspiratory muscles
As expiration ends, persisting air entrapment in a patient’s lungs creates positive end – expiratory pressure. This pressure compels the diaphragm at the beginning of its labors to carry out a major inspiratory effort in order to generate an inspiratory flow sufficing first to vanquish the positive pressure, and then to create the negative pressure that will provoke a flow of air into the lungs .
A large proportion of the energy that would normally be supplied by the diaphragm is expended in this laborious effort, which diminishes the volume of the respiratory reserve that can no longer easily meet the respiratory demand manifested during the patient’s struggles . His labors lead to the arrival of thoraco-pulmonary distension along with a tendency towards a flattening of diaphragmatic muscle fibers placing them in mechanically unfavorable conditions. More particularly, the diaphragmatic cupolas are lowered and flattened. As their contractile fibers are shortened, they become functionally insufficient. As a result, the inspiratory action of the diaphragm is weakened, and the accessory inspiratory muscles are solicited .
In order to adapt to these modifications, the diaphragm undergoes a diminishment in the number and length of its sarcomeres so as to recreate a more workable length-tension relationship at the level of the previously mentioned fibers . The strength produced by the new length is equal to that which had been produced by the old length. The peak inspiratory flow rate (PIFR) may thereby be conserved in many patients. On the other hand, ventilation takes place at a threshold level likely to lead to muscle fatigue. Type IIA diaphragmatic muscle fibers, which are less prone to fatigue, will tend to grow . The diaphragm’s aerobic capacities will likewise grow through increased capillarisation and more mitochondria .
As the disease evolves, the new length-tension relationship no longer allows the diaphragm to generate optimal strength when it contracts. As it labors, it approaches its maximum shortening capacity; as a result, its workload increases, as does its oxygen consumption, which becomes six times as great as that observed in normal subjects . As the disease worsens, it can no longer meet the heightened demand for oxygen, which is likewise augmented by the solicitation of accessory respiratory muscles , which appears to conserve their strength and endurance. All told, these different modifications of ventilatory mechanics lead to lowered inspiratory efficiency .
This evolution justifies today’s consensus according to which the decompensation undergone by the CPOD patient is largely due to muscle fatigue in the diaphragm and among the other respiratory muscles .
1.1.2
The expiratory muscles
Even though the abdominal muscles in humans appear to conserve their strength, their endurance is likely to diminish .
1.1.3
Flow-volume curve
The obstructive syndrome is accompanied by reduced respiratory flow even though volume remains the same or is only slightly modified. FEV1, the Tiffeneau ratio (FEV1/forced vital capacity) and the peak expiratory flow rate (PEFR) are all diminished.
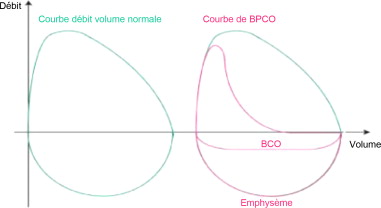
Due to the pathological conditions, the smaller bronchi are partially obstructed. The flow-volume curve or loop is typically concave towards the top, and its shape translates the normal expiratory velocity of the air in the large respiratory airways, and the reduced velocity in the smaller airways, which is due to their being partially obstructed ( Fig. 1 ).
Disease evolution is marked by increasing obstruction of the lower and smaller respiratory airways and by the irreversibility of the process .
Early therapeutic intervention during the Gold I and Gold II stages ( Table 1 ) is likely to be relatively efficient with regard to FEV1 decrease and quality of life, but relatively inefficient in terms of survival ( Fig. 2 ).
| Stages | Characteristics |
|---|---|
| 0 At risk | Chronic symptoms: coughing, expectoration FEV1/VC > 70% |
| I Non-severe COPD | FEV1/VC < 70% FEV1 > 80% of predicted figure with or without chronic symptoms (coughing, expectorations) |
| II Moderately severe COPD IIa IIb | FEV1/VC < 70% 30% < FEV1 < 80% of the predicted figure 50% < FEV1 < 80% of the predicted figure 30% < FEV1 < 50% of the predicted figure |
| III Severe COPD | FEV1/VC < 70% FEV1 < 30% of the predicted figure or FEV1 < 50% of the predicted figure with chronic respiratory insufficiency (PaO 2 < 60 mm Hg) |
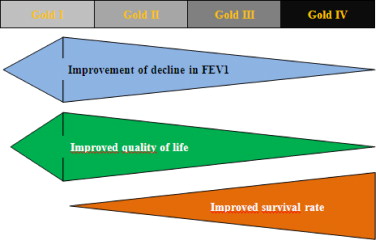
Later therapeutic intervention (Gold II and Gold III stages), once the obstruction would appear to be less reversible, will have more limited therapeutic efficiency, particularly with regard to FEV1 decrease. On the other hand, it may have a more pronounced effect on exacerbations and mortality. At these stages of disease evolution, comorbidities weigh heavily, and a global, integrated approach is consequently justified .
1.1.4
Rehabilitative care
From the standpoint of a rehabilitation technician, appropriate treatment of the respiratory symptoms linked with COPD (or with exacerbation of the latter) includes:
- •
bronchial decluttering meant to clear the airways;
- •
diaphragmatic rehabilitation (solicitation of the physiological diaphragmatic contraction) aimed at improved stamina;
- •
reinforcing of the lower limb muscles so as to limit functional deconditioning;
- •
psychological support and therapeutic education.
Weakened respiratory muscles and/or altered diaphragmatic mechanics may render the respiratory difficulty a key element in the clinical picture. Dyspnea and intolerance to physical exertion further worsen the quality of life .
While it appears that targeted training of the inspiratory muscles with specific loading (hand-held devices) may significantly augment their strength and endurance , the effects of this type of training on the other parameters have yet to be assessed. This situation has led us to implement a protocol aimed at reinforcing the respiratory muscles in conjunction with the application of bronchial decluttering techniques (accelerated expiratory flow and diaphragmatic rehabilitation). We have attempted to assess its effects as concerns:
- •
increased functional capacities of these muscles;
- •
mechanically improved diaphragmatic conditions;
- •
increased capacity for physical exertion.
Our hypothesis is that this type of training will facilitate:
- •
improved functioning of internal mechanics through ameliorated elastic behavior of the lungs;
- •
increased chest expansion through heightened enablement of the respiratory muscles;
- •
improved mechanical performance of the diaphragm;
- •
diminished dyspnea;
- •
improvement pertaining to the quality of life signs listed in Saint-George’s respiratory questionnaire (SGRQ).
Our hypothesis is that these improvements would be greater than those objectified when bronchial decluttering and diaphragmatic rehabilitation techniques are applied alone.
1.2
Population and methods
1.2.1
Population
Our prospective study was carried out over a period of 7 months. The subjects were selected subsequent to a survey conducted in a population of active and passive smokers and ex-smokers. Spirometric tests were proposed to patients presenting with clinical signs of COPD (coughing, dyspnea, expectorations) so as to validate or invalidate the clinical picture. The patients diagnosed as “COPD subjects” were divided into four different groups by assigning them, in the order of consultation and one by one, to groups 1 through 4. Patients were selected in accordance with the following inclusion criteria:
- •
cooperating COPD subjects;
- •
diagnosed clinically and through spirometric measurement (grades I and II in the Gold classification);
- •
presenting with 50% < FEV1 < 80% of predicted or theoretical value in the spirometric test;
- •
presenting with an improvement < 15% of the FEV1 following use of bronchodilators;
- •
from 45 to 75 years of age;
- •
of either sex.
Inclusion criteria were:
- •
heart failure or associated cardiac pathology;
- •
previous pulmonary or cardiac surgery;
- •
patient depending on oxygen therapy or undergoing cortisone treatment;
- •
associated neuromuscular pathologies.
The groups participating in the study were defined as follows ( Fig. 3 ):
- •
group 1 (inspiratory);
- •
group 2 (expiratory);
- •
group 3 (inspiratory and expiratory);
- •
group 4 (controls).
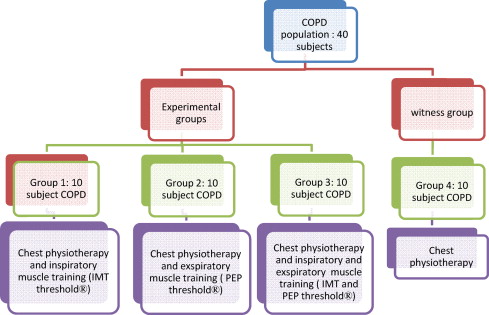
- •
Population: 40 COPD subjects.
- •
Experimental groups; control group.
- •
Group 1, 10 COPD subjects, respiratory physiotherapy and inspiratory muscle exercise (Threshold ® IMT).
- •
Group 2, 10 COPD subjects, respiratory physiotherapy and expiratory muscle exercise (Threshold ® PEP).
- •
Group 3, 10 COPD subjects, respiratory physiotherapy and inspiratory and expiratory muscle exercise (Threshold ® IMT and PEP).
- •
Group 4, 10 COPD subjects, respiratory physiotherapy.
Our variables comprised:
- •
FEV1, PEFR and peak instantaneous inspiratory flow rate (PIIFR) evaluated by spirometric measurement;
- •
quality of life signs evaluated by SGRQ and its secondary criteria (symptoms, repercussions on physical activity, impact on daily life);
- •
the distance covered during the 6-minute walking test;
- •
heart rate (HR) at rest, evaluated by a blood pressure monitor;
- •
dyspnea evaluated by the Sadoul scale;
- •
lower limb fatigue evaluated by the Visual Analog Fatigue Scale (VAFS);
- •
costo-diaphragmatic recess evaluated by frontal chest radiograph;
- •
strength developed by the inspiratory and expiratory muscles through Threshold ® measurement of maximum inspiratory (Ip max ) and expiratory (Ep max ) pressure.
1.2.2
Protocols
Sixteen rehabilitation sessions were carried out by each patient at a frequency of two sessions a week over the course of 8 weeks. The 1st and the 16th sessions were reserved for application of the different measurements and evaluations.
1.2.2.1
Evaluation protocol
It includes:
- •
measurement of the spirometric volumes according to the following three terms:
- ∘
maximum FEV1 (mFEV1),
- ∘
the PEFR reached in forced expiration initiated with maximum inspiration,
- ∘
the PIFR reached during a forced inspiratory vital capacity maneuver (FIVC) ;
- ∘
- •
assessment of the patient’s quality of life was carried out with SGRQ, which is an instrument expressly addressed to patients suffering from respiratory pathology. It includes 50 questions covering three dimensions: (1) symptoms, (2) repercussions on physical activity, (3) impact on daily life. The overall score summarizes the information taken as a whole. It ranges from 0 (the best) to 100 (the worst). The questionnaire dealt with the 2 most recent months, rather than the 12 months covered in the original version;
- •
evaluation of the distance (in meters) covered by the patient in 6 minutes . Once completed, this test allows for assessment of:
- ∘
the heart rate as measured by a heart rate monitor,
- ∘
the pauses during the test are counted,
- ∘
the fatigue of the lower limb muscles is based upon what the patients feels during the test and is measured in terms of the VAFS, which is a scale ranging from 0 (no tiredness) to 10 (intolerable tiredness necessitating rest and cessation of walking);
- ∘
- •
dyspnea is evaluated by the therapist according to what the patient feels during the test. It ranges from 0 to 4 on the Sadoul scale. Any diminution in the degree of dyspnea tends toward “0” and denotes improved functioning of the subject’s respiratory tract ;
- •
evaluation of maximal respiratory pressure is carried out in a seated position. The subject is provided with a nose clip. We carried out three measurements and retained the most favorable:
- ∘
maximal inspiratory pressure (Ip max as evaluated by Threshold ® IMT): the patient must carry out maximal forced inspiration following maximal expiration,
- ∘
maximal expiratory pressure (Ep max as evaluated by Threshold ® PEP): the patient must carry out a “quick and strong” expiration following deep inhalation,
- ∘
any increase in Ip max and Ep max denotes improved strength of the subject’s inspiratory and expiratory muscles;
- ∘
- •
pre-test/post-test comparison of the lower costo-diaphragmatic recess of the diaphragmatic cupola on a frontal chest radiograph in a standing position (with deep inhalation) ( Fig. 4 ).
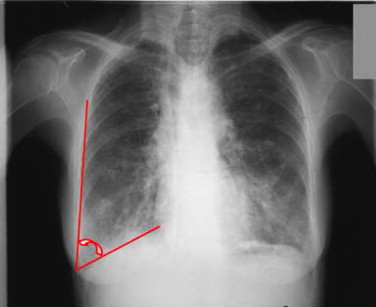
Fig. 4
Degree of flattening of the diaphragmatic cupola during maximal inspiration on a frontal radiograph. Origin: Frontal X-ray of a chronic obstructive pulmonary disease (CPOD) patient.
1.2.2.2
Measurement apparatuses
The EasyOne™ spirometer measures the flow of air entering into and exiting out of the patient’s lungs.
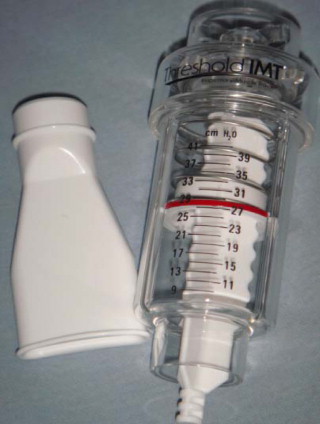
The Threshold ® IMT ( Fig. 5 ) is a tool helping to train the inspiratory muscles with a pressure load representing a fraction of the patient’s Ip max .
The Threshold ® PEP ( Fig. 6 ) is a tool used in expiratory rehabilitation by positive expiratory pressure, and it is equipped with a one-way valve functioning independently from the patient’s respiratory flow.
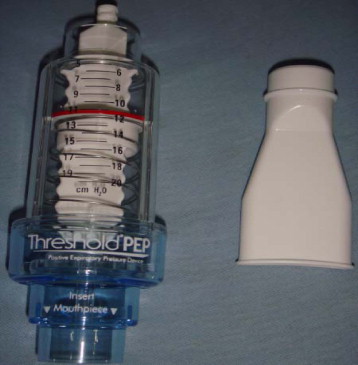
Pressure is adjustable and ranges from 4 cm H 2 O to 20 cm H 2 O. When the strength of the expiratory muscles is measured, it becomes possible to calculate Ep max .
The heart rate monitor automatically displays a heart rate reading.
The VAFS allows the patient to self-evaluate the fatigue experienced at the level of the lower limbs on a scale graded from 0 (no tiredness) to 10 (intolerable tiredness necessitating rest).
The SGRQ allows for assessment of the quality of life parameters.
The Sadoul scale classifies patients in terms of the stages of their pathology, which are graded from 0 (no dyspnea) to 4 (dyspnea accompanying even the slightest effort).
A simple goniometer with plastic arms enabled us to measure the costo-diaphragmatic recess on the frontal lung X-ray taken during maximal inspiration.
1.2.2.3
Experimental protocols
All of the patients participating in our study benefited from a rehabilitation program (group 4 protocol or control group protocol) involving:
- •
decluttering, or bronchial clearance, by means of Expiratory Flow Acceleration (EFA) or total slow expiration with glottis opened in lateral posture (ELTGOL);
- •
diaphragmatic rehabilitation led to awareness of increased abdominal volume during inspiration and a widening of the abdomen during expiration in the different positions at rest (seated, half-seated or standing) or involving effort (walking, toilet, stairs…) ;
- •
training the lower limb muscles: active flexion/extension exercises (bicycle) and stamina or endurance exercises (treadmill);
- •
psychological support program (cognitive-behavioral therapy techniques) aimed at diminishing the possible psychological pain experienced by patients and their friends and family;
- •
targeted education devoted especially to cessation of smoking and avoidance of polluting factors (second-hand smoking, industrial contaminants).
In addition to this protocol, the three experimental groups benefited from the following supplementary respiratory exercise protocols:
- •
group 1 (inspiratory) benefited from a rehabilitation protocol involving the inspiratory muscles, utilizing Threshold ® IMT, and consisting in eight to 10 2-minute cycles;
- •
group 2 (expiratory) benefited from a rehabilitation protocol involving the expiratory muscles, utilizing Threshold ® PEP, and consisting in eight to 10 2-minute cycles;
- •
group 3 (inspiratory and expiratory) benefited from a rehabilitation protocol involving the inspiratory muscles (Threshold ® IMT) and the expiratory muscles (Threshold ® PEP) and consisting in four or five 2-minute cycles devoted to each.
In order to proceed, an initial assessment of these groups was carried out by measuring “Ip max ” and “Ep max ” for each patient. The respiratory pressure load employed at the outset of the sessions represented 30% of each of the two parameters (Ip max and Ep max ). The pressure load was gradually raised to 60% of these parameters as the strength of the patient’s inspiratory and/or expiratory muscles increased. Exercise duration for each group went up little by little, and reached 20 to 30 minutes.
We utilized the statistical software XIstat 2009. Comparisons of means were carried out by a Student’s t test. We set the significance threshold at P < 0.05.
1.3
Results
1.3.1
Synoptic patient data
Forty COPD subjects participated in this study. The participants were women (52.5%, n = 21) and men (47.5%, n = 19). Their average age was 60.38 ± 8.02 years (45, 75). Twenty-seven subjects (70%) were smokers, eight subjects (20%) had stopped smoking over the past 6 months, and five subjects (8%) were non-smokers. The Body Mass Index (BMI) of these patients was 28.3 ± 3.11 kg/m 2 [22.2, 36.7].
1.3.2
Spirometric measurements
Table 2 shows significant FEV1 and PEFR improvement in group 1 alone. In none of our four groups was the PIFR modified.
| Spirometric measurements | Group 1 | Group 2 | Group 3 | Group 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-test | Post-test | P | Pré-test | Post-test | P | Pré-test | Post-test | P | Pré-test | Post-test | P | |
| FEV1 | 0.93 ± 0.39 | 1.44 ± 0.57 | 0.03 | 0.86 ± 0.44 | 0.04 ± 0.36 | > 0.05 | 1.06 ± 0.55 | 1.06 ± 0.47 | > 0.05 | 0.98 ± 0.32 | 0.9 ± 0.47 | > 0.05 |
| PEFR | 0.57 ± 0.14 | 0.76 ± 0.16 | 0.01 | 0.98 ± 0.45 | 1.25 ± 0.56 | > 0.05 | 1.33 ± 0.71 | 1.44 ± 0.65 | > 0.05 | 1.27 ± 0.44 | 1.15 ± 0.59 | > 0.05 |
| PIFR | 1.5 ± 0.72 | 1.79 ± 0.76 | > 0.05 | 1.13 ± 0.82 | 1.27 ± 0.46 | > 0.05 | 1.43 ± 0.8 | 1.36 ± 0.56 | > 0.05 | 1.57 ± 0.66 | 1.39 ± 0.58 | > 0.05 |
1.3.3
Quality of life
Table 3 shows significant improvement in the overall score pertaining to quality of life (symptoms experienced, repercussions on physical activity, impact on daily life) in the four groups constituting our population.
| Saint-George’s respiratory questionnaire | Group 1 | Group 2 | Group 3 | Group 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-test | Post-test | P | Pre-test | Post-test | P | Pre-test | Post-test | P | Pre-test | Post-test | P | |
| Symptoms | 41.68 ± 8.73 | 31.23 ± 7.93 | 0.013 | 41.05 ± 7.7 | 31.67 ± 8.16 | 0.015 | 39.04 ± 8.69 | 34.16 ± 8.5 | 0.01 | 43.3 ± 8.73 | 35.5 ± 7.88 | > 0.05 |
| Activity | 20.06 ± 2.45 | 17.11 ± 2.45 | 0.014 | 21.02 ± 2.45 | 16.98 ± 2.27 | 0.009 | 20.05 ± 2.38 | 17.18 ± 2.43 | 0.01 | 20.09 ± 2.45 | 16.98 ± 2.27 | 0.016 |
| Impact on daily life | 40.75 ± 7.7 | 31.23 ± 8.16 | 0.015 | 40.99 ± 8.73 | 34.65 ± 8.12 | > 0.05 | 31.23 ± 8.16 | 28.14 ± 8.5 | > 0.05 | 37.77 ± 7.39 | 32.1 ± 6.75 | > 0.05 |
| Total | 34.16 ± 7.09 | 25.92 ± 6.12 | 0.012 | 36.31 ± 6.9 | 29.34 ± 7.35 | 0.04 | 33.35 ± 5.28 | 27.19 ± 5.4 | 0.02 | 35.38 ± 4.72 | 28.13 ± 5.71 | 0.029 |
1.3.4
Functional capacities and fatigue
Table 4 details the results gathered during the 6-minute walking test (walking distance, heart rate, dyspnea and inspiratory muscle fatigue). It objectifies significant improvement in the dyspnea and lower limb fatigue scores in the four groups constituting our population. Only in group 1, however, did walking distance and heart rate significantly improve.
| 6-minute walking test | Group 1 | Group 2 | Group 3 | Group 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-test | Post-test | P | Pre-test | Post-test | P | Pre-test | Post-test | P | Pre-test | Post-test | P | |
| Walking distance (m) | 383 ± 27.1 | 413.5 ± 5.61 | 0.019 | 381 ± 45.57 | 405 ± 37.19 | > 0.05 | 368 ± 70.05 | 389 ± 63.85 | > 0.05 | 356 ± 47.66 | 388.5 ± 49.78 | > 0.05 |
| Heart rate (bpm) | 87.4 ± 8.4 | 85.5 ± 4.4 | 0.001 | 90.7 ± 7.47 | 85.5 ± 4.4 | > 0.05 | 88.3 ± 9.92 | 87.8 ± 9.3 | > 0.05 | 85.9 ± 8.13 | 86.2 ± 7.79 | > 0.05 |
| Dyspnea | 3.1 ± 0.74 | 1.2 ± 0.79 | 0.001 | 3 ± 0.67 | 1.5 ± 0.7 | 0.001 | 2.9 ± 0.99 | 1.3 ± 0.95 | 0.002 | 3.2 ± 0.63 | 1.8 ± 0.63 | 0.001 |
| Im fatigue | 4.9 ± 1.66 | 2.1 ± 1.6 | 0.001 | 5.1 ± 1.85 | 2.7 ± 1.64 | 0.007 | 4.1 ± 1.85 | 1.6 ± 1.84 | 0.007 | 4.4 ± 1.35 | 2.3 ± 1.06 | 0.001 |
1.3.5
The costo-diaphragmatic recess X-ray
In none of our four groups did we detect any modification of the lower left costo-diaphragmatic recess; this finding underscores how difficult it is to detect modified positioning of the diaphragm.
1.3.6
Respiratory pressures
Table 5 shows that the only significant improvements of the parameters pertaining to respiratory pressures were registered in groups 1 and 3 with regard to Ip max alone.
| Respiratory pressures | Group 1 | Group 2 | Group 3 | Group 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-test | Post-test | P | Pré-test | Post-test | P | Pré-test | Post-test | P | Pré-test | Post-test | P | |
| Ip max | 24 ± 4.55 | 29.8 ± 4.13 | 0.008 | 28.8 ± 1.99 | 32.4 ± 1.9 | 0.001 | ||||||
| Ep max | 18.8 ± 1.14 | 20 ± 0.67 | > 0.05 | 18 ± 1.14 | 19.9 ± 0.74 | > 0.05 | ||||||
1.4
Discussion
1.4.1
Spirometric volumes
Table 6 recapitulates the different individual and mean measurements carried out in our patients and allows us to draw attention to some of the points we consider important.
| Age | FEV1 | FEV1 | PEFR | PEFR | PIFR | PIFR |
|---|---|---|---|---|---|---|
| Pre-test | Post-test | Pre-test | Post-test | Pre-test | Post-test | |
| Group 1 | ||||||
| 53 | 0.86 | 1.77 | 0.69 | 0.66 | 1.63 | 2.88 |
| 62 | 1.34 | 1.12 | 0.66 | 0.74 | 2.56 | 0.82 |
| 70 | 0.69 | 1.15 | 0.39 | 0.76 | 0.37 | 1.86 |
| 66 | 0.58 | 0.37 | 0.35 | 0.38 | 1.76 | 0.98 |
| 67 | 0.93 | 1.08 | 0.70 | 0.76 | 1.35 | 0.97 |
| 45 | 0.52 | 1.82 | 0.63 | 0.79 | 0.71 | 2.13 |
| 63 | 0.71 | 1.18 | 0.41 | 0.96 | 1.13 | 1.45 |
| 68 | 1.56 | 1.67 | 0.73 | 0.84 | 1.87 | 1.59 |
| 68 | 1.49 | 2.30 | 0.53 | 0.90 | 2.54 | 2.85 |
| 48 | 0.68 | 1.94 | 0.64 | 0.83 | 1.08 | 2.37 |
| 61 ± 9.32 | 0.93 ± 0.39 | 1.44 ± 0.57 | 0.57 ± 0.14 | 0.76 ± 0.16 | 1.5 ± 0.72 | 1.79 ± 0.76 |
| Group 2 | ||||||
| 64 | 0.49 | 0.91 | 1.88 | 1.00 | 0.16 | 1.71 |
| 68 | 0.87 | 0.58 | 0.88 | 0.83 | 1.26 | 0.47 |
| 66 | 0.81 | 0.51 | 0.83 | 0.54 | 0.62 | 0.84 |
| 68 | 1.17 | 0.87 | 0.92 | 1.10 | 1.94 | 1.60 |
| 69 | 1.57 | 0.90 | 1.58 | 1.35 | 1.77 | 1.29 |
| 51 | 0.40 | 1.09 | 0.36 | 1.50 | 0.28 | 1.36 |
| 68 | 0.73 | 0.90 | 0.84 | 1.12 | 0.57 | 0.91 |
| 58 | 0.94 | 0.77 | 0.81 | 0.91 | 2.57 | 1.42 |
| 64 | 1.15 | 1.43 | 1.19 | 2.53 | 1.21 | 1.92 |
| 55 | 0.50 | 1.11 | 0.60 | 1.68 | 0.99 | 1.23 |
| 63.1 ± 5.29 | 0.86 ± 0.44 | 0.04 ± 0.36 | 0.98 ± 0.45 | 1.25 ± 0.56 | 1.13 ± 0.82 | 1.27 ± 0.46 |
| Group 3 | ||||||
| 60 | 1.05 | 0.84 | 1.70 | 1.17 | 1.55 | 0.74 |
| 69 | 0.39 | 0.38 | 0.49 | 0.54 | 0.63 | 0.59 |
| 69 | 2.15 | 1.27 | 2.82 | 1.31 | 2.64 | 1.82 |
| 50 | 0.36 | 0.91 | 0.57 | 1.08 | 1.24 | 2.50 |
| 53 | 1.31 | 1.21 | 1.66 | 2.34 | 1.96 | 1.51 |
| 48 | 0.55 | 1.16 | 0.57 | 1.21 | 0.54 | 1.69 |
| 58 | 1.25 | 1.20 | 1.66 | 1.59 | 1.02 | 1.26 |
| 54 | 1.29 | 1.16 | 1.34 | 1.28 | 1.45 | 0.99 |
| 75 | 1.41 | 1.59 | 1.49 | 2.77 | 2.71 | 1.43 |
| 54 | 0.89 | 0.94 | 1.07 | 1.19 | 0.59 | 1.15 |
| 59.1 ± 9.30 | 1.06 ± 0.55 | 1.06 ± 0.47 | 1.33 ± 0.71 | 1.44 ± 0.65 | 1.43 ± 0.8 | 1.36 ± 0.56 |
| Group 4 | ||||||
| 62 | 0.86 | 0.74 | 1.14 | 0.81 | 1.12 | 1.38 |
| 48 | 0.86 | 0.50 | 1.83 | 1.48 | 1.43 | 0.42 |
| 54 | 0.46 | 0.50 | 0.64 | 0.49 | 1.46 | 1.31 |
| 57 | 0.79 | 0.70 | 0.95 | 0.84 | 1.25 | 2.42 |
| 46 | 1.26 | 2.00 | 1.92 | 2.46 | 1.16 | 2.09 |
| 53 | 0.66 | 0.53 | 0.68 | 0.64 | 1.05 | 0.79 |
| 65 | 0.94 | 0.68 | 1.00 | 0.97 | 1.04 | 1.05 |
| 55 | 1.28 | 1.24 | 1.59 | 1.67 | 3.06 | 1.35 |
| 68 | 1.46 | 1.17 | 1.72 | 1.29 | 2.25 | 1.69 |
| 73 | 1.26 | 0.94 | 1.31 | 0.90 | 1.97 | 1.44 |
| 58.1 ± 8.72 | 0.98 ± 0.32 | 0.9 ± 0.47 | 1.27 ± 0.44 | 1.15 ± 0.59 | 1.57 ± 0.66 | 1.39 ± 0.58 |
1.4.1.1
Forced expiratory volume in 1 second (FEV1)
We noted that parametric improvement in this functional test following rehabilitation and exercise of the respiratory muscles was not significant. Our findings are in agreement with elements in the literature such as the studies by Lacasse, 1997 and Mota et al., 2007 .
We nonetheless took note in the first group of a non-uniform but significant improvement with regard to the FEV1 parameters. This is in agreement with the results of the study by Crisafulli et al., 2007 who found that exercise of the inspiratory muscles was more beneficial than exercise of the expiratory muscles with regard to improvement of pulmonary functioning in COPD patients.
After analytical study of the results, it appears that the younger subjects show greater improvement than the older ones, and this is in agreement with Dusser, 2008 , who found that early therapeutic intervention (Gold I and Gold II) has a more pronounced impact on FEV1 diminution. In the other groups, FEM1 underwent no modification.
1.4.1.2
Peak Expiratory Flow Rate (PEFR)
In the first group (inspiratory muscle exercise), we noted significantly improved PEFR parameters. The improvement came to 97.7% in patients with a mean age of 48.67 ± 4.6 years ( n = 3) and to 14% in patients with a mean age of 66.28 ± 6.2 years ( n = 7). In the other groups, PEFR underwent no modification. Once again, this is agreement with the findings of Mota et al. and Crisafulli et al., 2007 .
1.4.1.3
Peak Inspiratory Flow Rate (PIFR)
No significant PIFR improvement was noted in our four groups of patients. Whatever the rehabilitation methods applied, they do not modify PIFR parameters in COPD patients. Our results are in accordance with those described in the literature . Only the subjects with an age below 51 years in group 1 ( n = 3) presented highly pronounced PIFR improvement (115%). It would consequently appear that inspiratory muscle exercise by means of Threshold ® IMT effectively contributes to improvement with regard to the spirometric signs, especially PIFR, which is closely connected with the functional capacity of the inspiratory muscles. Moreover, inspiratory muscle training is recommended by the French-language pneumology society (SPLF) as a means of COPD patient rehabilitation .
1.4.2
Saint-George’s respiratory questionnaire (SGRQ)
At the end of the rehabilitation program, significant improvement with regard to the quality of life parameters (patient satisfaction, functional capacities in daily life and heightened autonomy) was observed in all of our groups, particularly group 1.
These improvements have been mentioned by Wijkstra et al. following a home rehabilitation program, and also by Lacasse and Mota et al. .
1.4.3
The 6-minute walking test
At the end of the rehabilitation program, significant improvement with regard to the 6-minute walking test was observed in the four groups. It was observed for all the test parameters in group 1, but only for dyspnea and inspiratory muscle fatigue in the other groups.
Our results are in agreement with those reported by Redelmeier et al. , Weiner et al. , Derom et al. , Crowe et al. , as well as Perez and Guenard .
1.4.4
The lower costo-diaphragmatic recess
At the end of the rehabilitation program, measurements of the lower left costo-diaphragmatic angle had not been statistically modified; had such modification occurred, our hypothesis pertaining to modifications of the morphology of the diaphragm might have been corroborated. No significant difference in radiographic measurement before and after rehabilitation has been reported in COPD subjects.
1.4.5
Maximal respiratory pressures
At the end of the rehabilitation program, significant improvement in measured Ip max was observed in the 1st and the 3rd groups. No modification was undergone by Ep max .
The results of our study are in accordance with the data to be found in the literature. Specific and customized training employing Threshold ® IMT significantly improves Ip max and inspiratory muscle strength. As a result, Threshold ® IMT has been used in the retraining of COPD patients (Gold 1). Similar results have been noted in several other studies in which it has been observed that inspiratory muscle exercise utilizing this tool, in the framework of a program progressing from 30% to 60% of the patient’s Ip max , effectively complements the pulmonary rehabilitation program followed by the COPD patient.
In our study, the Ep max measurements carried out before and after rehabilitation in group 2 (training targeting the expiratory muscles) and group 3 (training targeting both the inspiratory and the expiratory muscles) underwent no modification, notwithstanding use of a Threshold ® PEP specifically targeting the expiratory muscles. The absence of change may be due to the fact that the maximum pressure provided by the apparatus (21 cm H 2 O) is similar to the figures recorded in our patients (18 and 18.5 cm H 2 O).
1.4.6
The limits of this study
One of the shortcomings of our work consisted in the fact that all of the evaluations were carried out and that all of the rehabilitation techniques were implemented by the same physiotherapist. The presence of another person performing evaluations of these patients might have been more suitable in order to avoid any possible personal influence with regard to the results of the assessments, especially in measurement of dyspnea and in the terms of the SGRQ parameters.
The relatively low population was due to the exigencies entailed by the limited time frame of our study. This population would subsequently grow as we recruited more subjects for the different groups.
1.5
Conclusion
Subsequent to application of our rehabilitation protocols and given the end results, we have concluded that training and exercise undertaken by COPD patients brings about significant improvement with regard to dyspnea and quality of life signs, whatever the rehabilitation program applied. It also allows for significant improvement with regard to the functional capacities and strength of the patients’ inspiratory and expiratory muscles. These improvements are associated with positive FEV1 evolution.
The best results in the different groups of patients have been observed in subjects of an average age ranging from 45 to 51 years. These subjects generally tend to feel “normal”, when the fact of the matter is that they present with pathological spirometric measurements and are unaware of the impact the pathology will have on their medium-range and long-range state of health. It can consequently be suggested that rehabilitation should take on a major role in early care and treatment of their COPD. Enhanced awareness of the interest of care taking in the initial stages would be likely to exert a beneficial influence with regard to the debilitating natural evolution of the disease.
In addition, it would be worthwhile to proceed to a long-term assessment of the effectiveness of our rehabilitation programs.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
2
Version française
2.1
Introduction
La broncho-pneumopathie chronique obstructive (BPCO) touche les personnes de plus de 40 ans fumeurs ou exposées à la fumée. C’est la cinquième cause de décès dans le monde, après l’infarctus du myocarde, les accidents vasculaires cérébraux, les infections respiratoires communautaires et la tuberculose. L’Organisation mondiale de la santé (OMS) stipule que cette mortalité devrait doubler en 2020 par rapport à 1990, et devenir la troisième cause de mortalité en raison de l’augmentation du tabagisme, principale cause de la BPCO, en particulier chez les femmes .
La BPCO, longtemps peu symptomatique, débute par une toux et une expectoration matinale, progressivement s’installe une dyspnée à l’effort, puis au repos, pouvant aller jusqu’à limiter les activités de la vie courante et représenter un handicap considérable avant de compromettre le pronostic vital.
Cette maladie impose, une prévention ciblée, un diagnostic précoce et une prise en charge adaptée et basée essentiellement sur la rééducation respiratoire. Les résultats des différentes méta-analyses confirment avec un haut niveau de preuve que la rééducation (désencombrement bronchique, rééducation et entraînement des muscles des membres inférieurs) appliquée chez les sujets BPCO améliore la dyspnée, la tolérance à l’effort et la qualité de vie. Elle réduit la fréquence et la durée des hospitalisations avec les conséquences financières qui en découlent.
Trois critères principaux permettent d’évaluer la dégradation des possibilités fonctionnelles du patient BPCO : le déclin du volume expiratoire maximal à la première seconde (VEMS), le déclin de la qualité de vie et le taux de la mortalité . La spirale de l’évolution mène à une dégradation majeure de la qualité de vie .
2.1.1
Muscles inspiratoires
À la fin de l’expiration, il persiste au niveau des poumons du patient un piégeage d’un certain volume d’air créant une pression positive résiduelle. Cette pression impose, de la part du diaphragme au début de son travail, un effort inspiratoire important pour générer un débit inspiratoire suffisant lui permettant, tout d’abord, de vaincre la pression positive avant de pouvoir créer une pression négative qui provoquera l’appel de l’air dans les poumons .
Une partie importante de l’énergie qui pourrait être fournie par le diaphragme est utilisée pour cette lutte. Cela diminue le volume de réserve inspiratoire qui pourrait répondre à la demande respiratoire lors de l’effort du patient . Ce travail provoque l’installation d’une distension thoraco-pulmonaire et une tendance à l’aplatissement des fibres musculaires diaphragmatiques qui les place dans des conditions mécaniquement défavorables. Les coupoles du diaphragme deviennent abaissées et aplaties. Elles seront placées dans une situation d’insuffisance fonctionnelle par raccourcissement de leurs fibres contractiles. De ce fait, l’action inspiratoire du diaphragme se réduit ce qui provoque une sollicitation des muscles inspirateurs accessoires .
Le diaphragme, pour s’adapter à ces modifications, va diminuer le nombre et la longueur des sarcomères pour recréer un meilleur rapport tension-longueur au niveau de ces fibres . La force produite par cette nouvelle longueur est égale à celle produite par l’ancienne longueur. Le DIP serait préservée chez un bon nombre des patients. En revanche, la ventilation se fait aux alentours des seuils de charges susceptibles de conduire à une fatigue musculaire. Les fibres musculaires diaphragmatiques de type IIA, moins fatigables, vont augmenter . Ses capacités aérobies seront également majorées par augmentation de sa capilarisation et du nombre de ses mitochondries .
Avec l’évolution, la nouvelle relation tension-longueur ne lui permet plus de générer des forces optimales lors de sa contraction. Il travaille proche de ses capacités maximales de raccourcissement. Cela a pour conséquence une augmentation de son travail, et donc de sa consommation de l’oxygène, atteignant cinq fois les valeurs observées chez les sujets normaux . Il ne peut plus répondre à une demande accrue de la consommation d’oxygène lorsque la maladie s’aggrave. La demande en oxygène est également augmentée par la sollicitation des muscles respiratoires accessoires . Ses derniers semblent conserver leurs forces et leurs endurances. L’ensemble de ses modifications de la mécanique ventilatoire induisent une réduction de l’efficacité inspiratoire .
Ainsi, se trouve justifié le consensus actuel qui ramène la décompensation du patient BPCO à une fatigue musculaire du diaphragme et des autres muscles respiratoires .
2.1.2
Muscles expiratoires
Chez l’Homme, bien que la force des muscles abdominaux semble être conservée, leur endurance serait diminuée .
2.1.3
Courbe débit–volume
Le syndrome obstructif s’accompagne d’une réduction des débits respiratoires alors que les volumes sont peu ou pas modifiés. Le VEMS et le rapport de Tiffeneau (VEMS/capacité vitale fonctionnelle) ainsi que le débit expiratoire de pointe (DEP) sont diminués.
Les petites bronches sont partiellement obstruées à cause des conditions pathologiques. La courbe de débit–volume est typiquement concave vers le haut ce qui traduit la normalité de la vitesse de l’expiration de l’air qui se trouve dans les grosses voies respiratoires et la réduction de celle des petites voies qui est du à leurs obstructions partielles ( Fig. 1 ).