Fig. 33.1
(a) Wear-induced synovitis (Type I) in hematoxylin-eosin (HE) staining (magnification ×100) and (b) under polarized light. The synovial tissue is characterized of macrophages and polynucleus giant cells. PE particles of about 2 μm2 are taken up in phagocytes, but particles of more than 5 μm2 in size are predominantly in polynucleus giant cells (Courtesy of M. Günther, Department of pathology, Hospital Brandenburg, Germany)
Type II:
Infection-induced type of synovitis (Fig. 33.2)
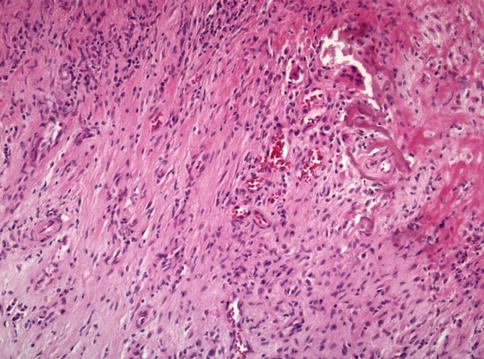

Fig. 33.2
Infection-induced type of synovitis (Type II) in HE staining (magnification ×100). The synovial tissue will provide information about high- and low-grade infection. The low-grade infection is dominated by fibroblasts, reactive vessel formation and neutrophil granulocytes, plasma cells, and small aggregation of lymphocytes. The tissue in high-grade infection shows significantly more neutrophil granulocytes (Courtesy of M. Günther, Department of pathology, Hospital Brandenburg, Germany)
Type III:
Mixed type of synovitis (Fig. 33.3a, b)
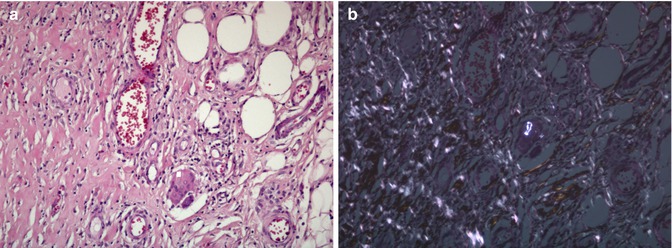

Fig. 33.3
(a) Mixed type of synovitis (Type III) in HE staining (magnification ×100) and (b) under polarized light. The tissue shows a combination of cells typically for both wear-induced and infection-induced synovitis (combination of type I and II) (Courtesy of M. Günther, Department of pathology, Hospital Brandenburg, Germany)
Type IV:
Indifferent type of synovitis (Fig. 33.4a, b)
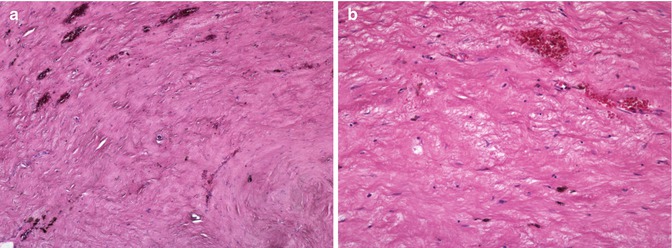

Fig. 33.4
(a) Indifferent type of synovitis (Type IV) in HE staining under magnification of ×40 and (b) magnification of ×100. The indifferent type of synovitis does not show the typical wear-induced or infection-induced cells. The indifferent type of synovitis is rather characterized by collagen connective tissue. The tissue is frequently covered by synovial-like membrane (Courtesy of M. Günther, Department of pathology, Hospital Brandenburg, Germany)
The allergy to implants can be considered a special histopathological form, which includes a lymphocyte infiltration and a positive allergy test. No typical cells such as seen in type I and type II are present.
The periprosthetic interface membrane should be considered as reference tissue for histological diagnosis of PJI.
The problem is that, without removal of the TKR, the periprosthetic membrane is not regularly accessible [12, 21, 41]. Microbiological investigations of joint aspirations and tissue samples can yield false-negative results in 28 and 14 %, respectively, when obtained surgically without removal of the TKR [27]. Histopathological analysis of periprosthetic tissue differentiates more precisely between aseptic and septic loosening.
Intraoperative examination of polymorphonuclear cells in frozen-section samples in TKR revision surgery is highly reliable. When the test is positive, the TKR is infected. However, when it is negative, the final decision of the surgeon should rely on other preoperative and intraoperative tests [9].
Intraoperative frozen-section histology is reasonably efficient and reliable, and is rapidly available for distinguishing between aseptic and septic loosening.
Samples should be obtained from highly suspicious areas and divided into two fragments, one for culture and the other for histology. The samples are then frozen in liquid nitrogen, cut, and dyed with hematoxylin-eosin (HE).
Intraoperative frozen-section histology is reasonably efficient and reliable, and is rapidly available for distinguishing between aseptic and septic loosening. A diagnosis can be provided within 15–30 min. Histological grading as proposed by Mirra et al. includes the number and type of inflammatory cells and suggests that the cutoff point for the histological diagnosis of infection is five polymorphs per high-power field [24]. This is in agreement with Feldman et al., who reported a sensitivity of 100 % and a specificity of 96 % for the detection of infection. Another study highlighted that this number is too high, and a considerable number of low-grade infections would be missed [2].
The positive predictive value of intraoperative frozen sections could be increased if an index of 10 rather than 5 polymorphonuclear leukocytes per high-power field is applied [22]. Then a sensitivity of 84 %, a specificity of 99 %, and a positive predictive value of 89 % was found [22]. Furthermore, the sensitivity may be enhanced if the surgeon obtains multiple small tissue samples and if more than two fields are then analyzed. Five samples should be taken from different sites. False-positive frozen sections may be due to the following causes:
False-negative results may be due to a different selection of samples for culture and frozen sections [22].
In summary, the analysis of intraoperative frozen sections is an accurate aid in the decision-making process during revision surgery. It can be used with good confidence at revision TKR. Lonner et al. recommended the removal of TKR and staged reimplantation if there are at least ten polymorphonuclear leukocytes per high-power field [22]. Primary exchange of the implant is performed if there are less than five polymorphonuclear leukocytes per high-power field. If there are five to nine polymorphonuclear leukocytes per high-power field, it may depend on the finding during surgery and the surgeon’s experience whether one-stage or two-stage revision surgery is performed (Figs. 33.5 and 33.6).
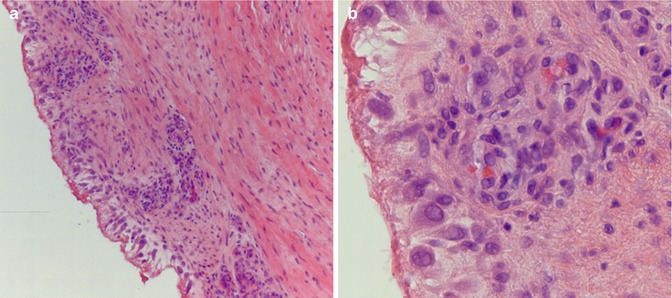
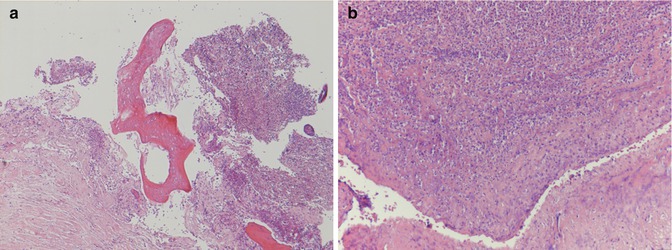

Fig. 33.5
(a) Chronic aseptic arthritis in HE staining (low-power magnification, courtesy of João Cruz, MD, MSc, Pathology Department, Hospital CUF Descobertas, Lisbon, Portugal). (b) Mild chronic and reactive exudative process with scattered lymphocytes present. No polymorphonuclear neutrophils were detected (hematoxylin-eosin staining, high-power magnification) (Courtesy of João Cruz, MD, MSc, Pathology Department, Hospital CUF Descobertas, Lisbon, Portugal)

Fig. 33.6
(a) Severe infectious arthritis (hematoxylin-eosine staining, low-power magnification, courtesy of João Cruz, MD, MSc, Pathology Department, Hospital CUF Descobertas, Lisbon, Portugal). (b) Heavy fibrino-granulocytic inflammatory exudation (above) and eroded articular surface (below) (hematoxylin-eosin staining, high-power magnification) (Courtesy of João Cruz, MD, MSc, Pathology Department, Hospital CUF Descobertas, Lisbon, Portugal)
In TKR revision surgery the presence of polymorphonuclear cells correlates well with infection, but their absence does not exclude it. Thus, frozen-section analysis should be included in the diagnostic protocol at revision TKR.
33.3.3 Leukocyte Esterase Test
Finally, the leukocyte esterase test has been proposed for diagnosing infection. Leukocyte esterase is an enzyme secreted by neutrophils that are present at the site of infection. Parvizi et al. investigated a colorimetric strip test for this enzyme in the synovial fluid. The change of color indicates the activity of active leukocyte esterase (graded as negative, trace, +, ++). It is noted after 1 or 2 min. This test has been used since the early 1980s to detect urinary tract infections.
Intraoperative frozen-section histology is reasonably efficient and reliable, and is rapidly available for distinguishing between aseptic and septic loosening.
When a ++ reading was considered positive, the leukocyte esterase test was 80.6 % sensitive, 100 % specific, with a positive predictive value of 100 %, and a negative predictive value of 93.3 % [35]. The leukocyte esterase level correlated strongly with the percentage of polymorphonuclear leukocytes and total white blood cell count in the aspirate as well as with the erythrocyte sedimentation rate and CRP level in the serum [35].
The ease, speed, and inexpensiveness of the leukocyte esterase test enable it to become an important adjunctive diagnostic tool for the orthopedic surgeon. A ++ reading confirms the diagnosis of PJI with relative certainty, and a negative or trace reading allows the surgeon to almost entirely rule out PJI. It can be used by itself or in conjunction with other tests, either as a rapid screening mechanism or for confirmation of a PJI pre- or intraoperatively [35].
References
1.
Abdul-Karim FW, McGinnis MG, Kraay M. Frozen section biopsy assessment for the presence of polymorphonuclear leukocytes in patients undergoing revision of arthroplasties. Mod Pathol. 1998;11:427–31.PubMed
2.
Athanasou NA, Pandey R, Steiger R, Crook D, McLardy-Smith P. Diagnosis of infection by frozen section during revision arthroplasty. J Bone Joint Surg Br. 1995;77:28–33.PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








