The Cervical Spine
Randall T. Loder
Many of the diseases and congenital anomalies affecting the pediatric cervical spine are simply a reflection of aberrant growth and developmental processes. This chapter discusses these diseases and anomalies in this framework. A basic knowledge of the normal embryology, growth, and development of the pediatric cervical spine is necessary to understand these conditions. Most of the anomalies and diseases involving the pediatric cervical spine are easily divided into those of the upper (occiput, C1, C2) and lower (C3-C7) segments.
NORMAL EMBRYOLOGY, GROWTH, AND DEVELOPMENT
Embryology
Occipitoaxioatlas Complex.
The occiput is formed from at least four or five somites. All definitive vertebrae develop from the caudal sclerotome half of one segment and the cranial sclerotome half of the succeeding segment (1). These areas of primitive mesenchyme separate from each other during fetal growth and then undergo chondrification and subsequent ossification. This chondrification and ossification is a passive process, following the blueprint laid down by the mesenchymal anlage. Because of this sequencing, the cranial half of the first cervical sclerotome remains as a half segment between the occipital and the atlantal rudiments and is known as the proatlas. The primitive centrum of this proatlas becomes the tip of the odontoid process, whereas its arch rudiments assist in the formation of the occipital condyles (2). The vertebral arch of the atlas separates from its respective centrum, becoming the ring of C1; the separated centrum fuses with the proatlas above and the centrum of C2 below, to become the odontoid process and body of C2. The axis forms from the second definitive cervical vertebral mesenchymal segment. The odontoid process is the fusion of the primitive centra of the atlas and the proatlas half segment. The posterior arches of C2 form from only the second definitive cervical segment.
Thus, the atlas is made up of three main components: the body and the two neural arches. The axis is made up of four main components: the body, two neural arches, and the odontoid (or five components if the proatlas rudiment is considered) (Figs. 21-1 and 21-2).
Vertebrae C3-C7.
These vertebrae follow the normal formation schema of all vertebrae (3). A portion of the mesenchyme from the sclerotomal centrum creates two neural arches that migrate posteriorly and around the neural tube. This eventually forms the pedicles, the laminae, the spinous processes, and a very small portion of the body. The majority of the body is formed by the centrum. An ossification center develops in each of the two neural arches and one in the vertebral center, with a synchondrosis formed by the cartilage between the ossification centers.
Basic Science, Embryology, and Gene Expression.
In the past decade, there has been an explosion of knowledge regarding the human genome and how it relates to normal developmental processes and pathologic conditions. Vertebral segmentation begins with clustering segments of the paraxial mesoderm, the somites. Segmentation of the mesoderm into somites is an important yet fundamental process that allows for spatial specialization in the organism and is under genetic control.
The homeobox is a highly conserved 160-base pair sequence found in the homeobox genes, termed Hox genes for short. These Hox genes encode a highly conserved family of transcription factors that play fundamental roles in morphogenesis during embryonic development. Vertebrate Hox genes help control developmental patterning in the embryo along the primary (head-to-tail) and secondary (genital and limb bud) axes. There are 39 Hox genes in vertebrates that are organized into four clusters located on different chromosomes. In the human, these clusters are named HOXA, HOXB, HOXC, and HOXD, located on chromosomes 7p14, 17q21, 12q13, and 2q31, respectively (4). In animals, they are written in lower case (e.g., Hoxc); in the human, they are written in upper case (e.g., HOXC). Each cluster contains 9 to 11 genes, all oriented in the same 5′ to 3′ direction of transcription. There are 13 possible subsets of genes; no single cluster contains a representative from all 13 known numbered subsets (paralogous groups). The numbering of the genes in each cluster is based on their sequence similarity and relative positions, starting from that end of the complex that is expressed most anteriorly (cranially). The equivalent genes in each complex are called a paralogous group.
The expression domains of the HOX clusters display a nested arrangement. Along the body axis, the Hox genes are generally expressed with discrete rostral cutoffs that coincide with either existing or emergent anatomic landmarks. Hox genes at the end of the 3′ cluster (e.g., HOXA1) are generally expressed early, in anterior and proximal regions; Hox genes at the 5′ end (e.g., HOXA13) are generally expressed later, in more posterior and distal regions. Thus, the lower numbered Hox genes are involved in the development of the axial skeleton, and the higher numbered Hox genes are involved in the development of the limbs. There has been an explosion of knowledge regarding defects in the Hox genes and resultant congenital spinal anomalies in experimental animals, and to much lesser extent in humans (Table 21-1). The defect can be a distinct Hox gene mutation intentionally produced by the investigator or more random hits by teratogens (methanol, boric acid, retinoic acid, maternal hyperthermia) (13, 14, 15, 16 and 17).
Another group of genes, the Pax genes, are also integrally involved in vertebral development. The Pax genes are a highly conserved family of developmental control genes that encode transcription factors containing a 128-amino acid DNA-binding domain (18, 19), called the paired box (20). To date, there are nine PAX genes (12, 21). The Pax gene family is broken down into four subgroups (Pax1 and Pax9; Pax2, Pax5, and Pax8; Pax3 and Pax7; Pax4 and Pax6). Pax1 and Pax9 induce chondrogenic differentiation in the paraxial mesenchymal mesoderm of the sclerotome (19, 20, 22). Pax1 expression is also seen in the posterior occiput, indicating that the basilar occiput from a developmental standpoint can be considered the uppermost vertebra (23). Thus, they are critically involved in vertebral formation. Abnormalities in the PAX1 sequence in humans have been associated in some patients with Klippel-Feil syndrome (12).
The Hedgehog family of proteins has also become increasingly recognized as crucial to axial skeletal development. The best known of these proteins is the sonic hedgehog (shh), which is expressed in the notochord (24, 25). Shh is believed to be the signal for induction of the ventral somite to differentiate into the sclerotome (25). In shh knockout mice, most sclerotomal derivatives are absent, in conjunction with reduced expression of Pax1 (25). Thus, absence of shh leads to absence of Pax1 expression and subsequent failure of the mesenchymal cells to chondrify. Defective shh signaling during embryogenesis in mice results in anomalies similar to those seen in the human VACTERL association (26).
Growth and Development
Atlas.
Ossification is present only in the two neural arches at birth (27). These ossification centers extend posteriorly toward the rudimentary spinous process to form the posterior synchondrosis and anteriorly into the articular facet region to form all of the bone present in the facets. Anteromedial to each facet, the neurocentral synchondroses form, joining the neural arches and the body; this occurs on each side of the expanding anterior ossification center. The body starts to ossify between 6 months and 2 years, usually in a single center. By 4 to 6 years, the posterior synchondrosis fuses, followed by the anterior ones slightly thereafter. The final internal diameter of the pediatric C1 spinal canal is determined by 6 to 7 years of age. Further growth is obtained only by periosteal appositional growth on the external surface, which leads to thickening and an increased height, but without changing the size of the spinal canal. Thus, a spinal fusion after the age of 6 or 7 years has minimal impact on the internal canal diameter; when possible, surgical fusion should not be performed before this age due to the potential for later cervical stenosis.
TABLE 21.1 Axial Skeletal Malformations Due to Genetic Abnormalities | |||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Axis.
The odontoid develops two primary ossification centers that usually coalesce within the first 3 months of life; these centers are separated from the C2 centrum by the dentocentral synchondrosis (28, 29). This synchondrosis is below the level of the C1-C2 facets and contributes to the overall height of the odontoid as well as to the body of C2. It is continuous with the vertebral body and facets, and it coalesces with the anterior neurocentral synchondroses and finally at the dentocentral synchondrosis. This closure occurs between 3 and 6 years of age. The tip of the dens comprises a cartilaginous region similar to an epiphysis, the chondrum terminale, which develops an ossification center between 5 and 8 years, becoming the ossiculum terminale. The ossiculum terminale fuses to the remainder of the odontoid between 10 and 13 years of age.
The posterior neural arches are partially ossified at birth, joined by the posterior synchondrosis. By 3 months of age, these arches, growing more posteriorly, form the rudimentary spinous process. By 1 year of age, ossification fills the spinous process, and by 3 years of age, the posterior synchondrosis has fused. Thus, both the posterior and the anterior synchondroses are closed by 6 years of age, and there is no further increase in spinal canal size after this age.
C3-C7.
At birth, all three ossification centers are present. The anterior synchondrosis (i.e., neurocentral synchondrosis) is slightly anterior to the base of the pedicles; it usually closes between 3 and 6 years of age. The posterior synchondrosis is at the junction of the two neural arches; it usually closes by 2 to 4 years of age. In the neonate and the young child, the articular facets are horizontal but become more vertically oriented as the child ages and reaches the normal adult configuration. They are also more horizontal in the upper cervical spine than in the lower cervical spine. The vertebral bodies enlarge circumferentially by periosteal appositional growth, whereas they grow vertically by endochondral ossification. Secondary ossification centers develop at the tips of the spinous processes and the cartilaginous ring apophyses of the bodies around the time of puberty. These ring apophyses are involved in the vertical growth of the body. These secondary ossification centers fuse with the vertebral body around age 25 years. There is an overall increase in pedicle axis width but not length as the child grows; thus, pedicle screw fixation of the cervical spine in children is not considered safe (30).
Vertebral Body and Canal Diameter Changes with Growth.
Due to the fact that the immature vertebrae are more cartilaginous compared to the mature adult vertebrae, there are significant differences between normal vertebral measurements in the child compared to the adult (31). As the child ages, the vertebral body height increases relative to the vertebral body depth. This is due to the activity of the apophyseal end plates that contribute proportionally more growth to the height of the vertebral body compared to the appositional growth that contributes to the depth of the vertebral body. The vertebral body height-to-depth ratio increases from approximately 0.5 in children <1 year of age to 0.8 to 0.9 in adults. This remains relatively constant for all vertebral bodies from C3 to C7. With these changes in the vertebral body height relative to depth, there are also changes in the sagittal diameter of the canal relative to vertebral body depth. The ratio of the sagittal canal diameter to vertebral body depth is stable at 1.4 in children from birth to 7 to 8 years of age and then gradually decreases to the normal 1.0 adult value (31). Knowledge of these normal growth parameters is important when determining the possibility of platyspondyly or spinal stenosis.
Normal Radiographic Parameters.
Certain radio graphic parameters that indicate pathology of the cervical spine in adults represent normal developmental processes in children (32). These parameters are the atlantooccipital motion and atlanto-dens interval (ADI), pseudosubluxation and pseudoinstability, variations in the curvature of the cervical spine that may resemble spasm and ligamentous injury, variations in the presence of skeletal growth and growth centers that may resemble fractures, and anterior soft-tissue widening. Normal cervical spine motion in children is also discussed.
Atlanto-Dens Interval and Atlantooccipital Motion.
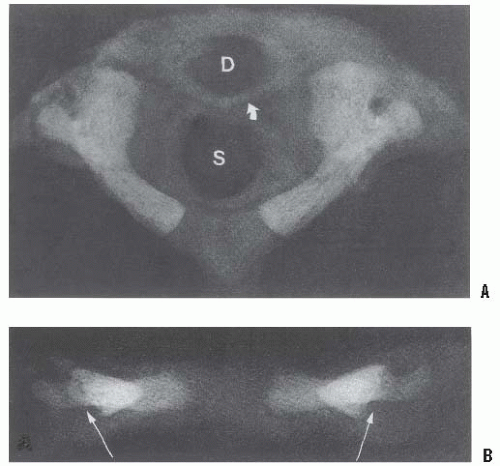
These intervals are determined on lateral flexion and extension views, which should be performed voluntarily with the patient awake. The ADI is the space between the anterior aspect of the dens and the posterior aspect of the anterior ring of the atlas (Fig. 21-3). An ADI of more than 5 mm on flexion and extension lateral radiographs indicates instability (33, 34). This is more than the 3-mm adult value because of the increased cartilage content of the odontoid and ring of the atlas in children as well as the increased ligamentous laxity in children. In extension, overriding of the anterior arch of the atlas on top of the odontoid also can be seen in up to 20% of children (35) (see Fig. 21-6B).
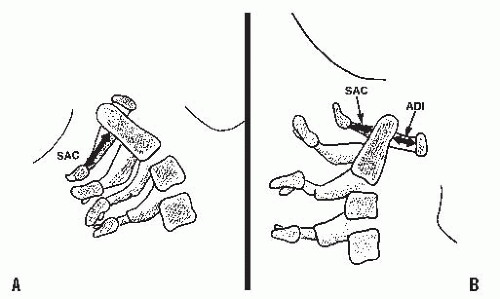
A mild increase in the ADI may indicate a subtle disruption of the transverse atlantal ligament. In adults, an ADI > 5 mm indicates ligament rupture (36). In chronic atlantoaxial conditions (e.g., rheumatoid arthritis, Down syndrome, congenital anomalies), the ADI is less useful. In children with these disorders who are frequently hypermobile but do not have ruptured transverse atlantal ligaments, the ADI is increased beyond the 3- to 5-mm range. The complement of the ADI, the space available for the cord (SAC), is a more useful measure in this situation. This space is the distance between the posterior aspect of the dens and the anterior aspect of the posterior ring of the atlas or the foramen magnum. A SAC of <13 mm may be associated with neurologic problems (37).
In patients in whom there is an attenuation of the transverse atlantal ligament without rupture, the alar ligament provides some stability. It acts like a checkrein (38), first tightening up in rotation and then becoming completely taut as the odontoid process continues to move posteriorly for a distance equivalent to its full transverse diameter. This safety zone between the anterior wall of the spinal canal of the atlas, the axis, and the neural structures is an anatomic constant equal to the transverse diameter of the odontoid. This constant defines Steel’s rule of thirds: one-third cord, one-third odontoid, and one-third space. This rule remains constant throughout the growth of the cervical spine (39). The cord can move into this space (safe zone) when the odontoid moves posteriorly because of an attenuated transverse atlantal ligament. It is here that the alar ligament becomes taut, acting as a checkrein and secondary restraint, preventing further movement of the odontoid into the cord. In the chronic situation, it is important to recognize when this safe zone has been exceeded and the child enters the region of impending spinal cord compression. In the case of trauma, the alar ligament is insufficient to prevent a fatal cord injury in the event of another neck injury similar to the one that caused the initial interruption of the transverse atlantal ligament.
Normal ranges of motion at the atlantooccipital joint are not well defined. In a series of 40 normal college freshman, the tip of the odontoid remained directly below the basion of the skull in both flexion and extension (40). Thus, the joint should not allow any horizontal translation during flexion and extension. Tredwell et al. (41) believe that a posterior subluxation of the atlantooccipital relation in extension of more than 4 mm indicates instability (Fig. 21-4). This can be measured
as the distance between the anterior margin of the condyles at the base of the skull and the sharp contour of the anterior aspect of the concave joint of the atlas anteriorly, or as the distance between the occipital protuberance and the superior arch of the atlas posteriorly. Another method to measure posterior subluxation of the atlantooccipital joint is that of Wiesel and Rothman (42) (Fig. 21-5). With this technique, occiput-C1 translation from maximum flexion to maximum extension should be no more than 1 mm in normal adults. These norms in children have not yet been established.
as the distance between the anterior margin of the condyles at the base of the skull and the sharp contour of the anterior aspect of the concave joint of the atlas anteriorly, or as the distance between the occipital protuberance and the superior arch of the atlas posteriorly. Another method to measure posterior subluxation of the atlantooccipital joint is that of Wiesel and Rothman (42) (Fig. 21-5). With this technique, occiput-C1 translation from maximum flexion to maximum extension should be no more than 1 mm in normal adults. These norms in children have not yet been established.
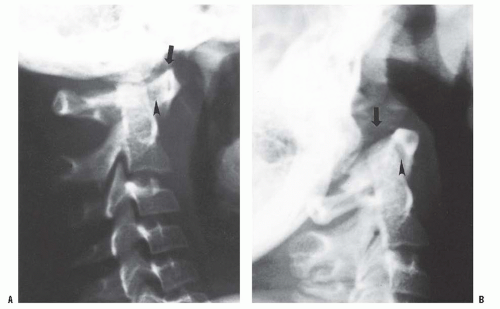 FIGURE 21-4. Lateral (A) flexion and (B) extension radiographs of an 11-year-old boy with Down syndrome. The child presented with loss of hand control when flexing his neck. Using the method of Tredwell et al. (41), the atlantooccipital distance is measured as the distance between the anterior margin of the condyles at the base of the skull and the sharp contour of the anterior aspect of the concave joint of the atlas. More than 4 mm of posterior translation is abnormal. The atlantooccipital distance (arrows) measured 10 mm in extension and 1 mm in flexion. The ADI was 1 mm in extension and 6 mm in flexion, for a total of 5 mm of motion (arrowheads). The SAC was 17 mm in flexion and 20 mm in extension. Both occipitoatlantal instability (more than 4 mm posterior translation) and atlanto-dens hypermobility (5 mm ADI in flexion) were present. |
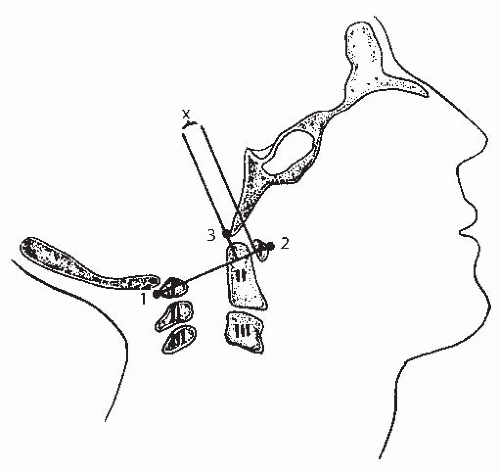 FIGURE 21-5. The method of measuring atlantooccipital instability according to Weisel and Rothman (42). The atlantal line joins points 1 and 2. A perpendicular line to the atlantal line is made at the posterior margin of the anterior arch of the atlas. The distance (x) from the basion (3) to the perpendicular line is measured in flexion and extension. The difference between flexion and extension represents the AP translation at the occipitoatlantal joint; in normal adults, this translation should be no more than 1 mm. (From Gabriel KR, Mason DE, Carango P. Occipito-atlantal translation in Down’s syndrome. Spine 1990;15:996-1002, with permission.) |
Pseudosubluxation.
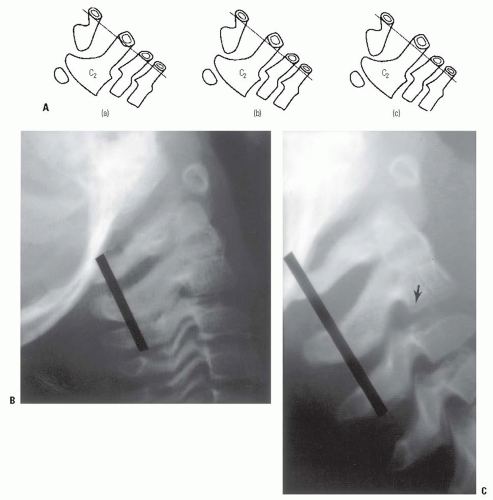
The C2-C3, and to a lesser extent, the C3-C4 interspace in children, have a normal physiologic displacement. In a study of 161 children (35), marked anterior displacement of C2 on C3 was observed in 9% of children between 1 and 7 years old. In a more recent study, 22% of 108 polytrauma children demonstrated pseudosubluxation, and had no association with intubation status or injury severity (43). In some children, the anterior physiologic displacement of C2 on C3 is so pronounced that it appears pathologic (pseudosubluxation). To differentiate this from pathologic subluxation, Swischuk (44) has used the posterior cervical line (Fig. 21-6) drawn from the anterior cortex of the posterior arch of C1 to the anterior cortex of the posterior arch of C3. In physiologic displacement of C2 on C3, the posterior cervical line may pass through the cortex of the posterior arch of C2, touch the anterior aspect of the cortex of the posterior arch of C2, or come within 1 mm of the anterior cortex of the posterior arch of C2. In pathologic dislocation of C2 on C3, the posterior cervical line misses the posterior arch of C2 by 2 mm or more.
The planes of the articular facets change with growth. The lower cervical spine facets change from 55 to 70 degrees, whereas the upper facets (i.e., C2-C4) may have initial angles as low as 30 degrees, which gradually increase to 60 to 70 degrees. This variation in facet angulation, along with normal looseness of the soft tissues, intervertebral discs, and the relative increase in size and weight of the skull compared with the trunk, are the major factors responsible for this pseudosubluxation. No treatment is needed for this normal physiologic subluxation.
Variations in the Curvature and Growth of the Cervical Spine That Can Resemble Injury.
In the classic study of Cattell and Filtzer (35), 16% of normal children showed a marked angulation at a single interspace, suggestive of injury to the interspinous or posterior longitudinal ligament; 14% showed an absence of the normal lordosis in the neutral position; and 16% showed an absence of the flexion curvature between the second and the seventh cervical vertebrae, which could be erroneously interpreted as splinting secondary to injury. These findings may occur in children up to 16 years of age.
Spina bifida of the posterior arch, or multiple ossification centers of the ring of C1, may mimic fractures. They can be delineated from fractures by their smooth cortical margins. In some children, the posterior ring of C1 remains cartilaginous, which is usually of no clinical significance (45, 46). Spina bifida also may occur at other cervical levels, and the overlapping lucent areas on anteroposterior (AP) radiographs when crossing a vertebral body, may mimic a vertical fracture of the body. Defects in the posterior arch of the atlas are present in 3.7% of the normal population (47).
The dentocentral synchondrosis of C2 begins to close between 5 and 7 years of age (28). However, it may be visible in vestigial forms up to 11 years of age (35) and may be erroneously interpreted as an undisplaced fracture. Similarly, the apical odontoid epiphysis (i.e., ossiculum terminale) may appear by 5 years of age, although it most typically appears around 8 years of age. This also can be misinterpreted as an odontoid tip fracture.
Wedging of the C3 vertebral body is a normal radiographic finding in 7% of younger children (Fig. 21-6B); the wedging corrects as the child matures and is extremely rare after age 13 (48). If it is unclear whether the wedging is a normal variation or a true compression fracture in the face of a traumatic history, a computed tomography (CT) scan will demonstrate fracture lines through the body if a fracture is present. In the lower cervical levels, secondary centers of ossification of the spinous processes may resemble avulsion fractures (35).
Normal Lower Cervical Spine Motion.
Generally, the interspinous distances increase with increasing age, being the smallest at C4-C5 and the largest at C6-C7, until 15 years
of age, when this distance is largest at C5-C6 (34). The AP displacement, from hyperflexion to hyperextension, decreases from C2-C3 to C6-C7. The angular displacement is greatest (15 degrees) at C3-C4 and C4-C5 for children 3 to 8 years of age, is greatest (17 degrees) at C4-C5 for children 9 to 11 years of age, and is greatest (15 degrees) at C5-C6 for children 12 to 15 years of age.
of age, when this distance is largest at C5-C6 (34). The AP displacement, from hyperflexion to hyperextension, decreases from C2-C3 to C6-C7. The angular displacement is greatest (15 degrees) at C3-C4 and C4-C5 for children 3 to 8 years of age, is greatest (17 degrees) at C4-C5 for children 9 to 11 years of age, and is greatest (15 degrees) at C5-C6 for children 12 to 15 years of age.
CONGENITAL AND DEVELOPMENTAL PROBLEMS
Torticollis.
Torticollis is a combined head tilt and rotatory deformity. Torticollis indicates a problem at C1-C2 because 50% of the cervical spine rotation occurs at this joint. A head tilt alone indicates a more generalized problem in the cervical spine.
The differential diagnosis of torticollis is large and can be divided into osseous and nonosseous types. In a recent large series from a tertiary care pediatric orthopaedic center (49), a nonmuscular etiology of torticollis was found in 18% of patients, most frequently the Klippel-Feil syndrome or a neurologic disorder (ocular pathology, or central nervous system lesion).
The differential diagnosis of torticollis is large and can be divided into osseous and nonosseous types. In a recent large series from a tertiary care pediatric orthopaedic center (49), a nonmuscular etiology of torticollis was found in 18% of patients, most frequently the Klippel-Feil syndrome or a neurologic disorder (ocular pathology, or central nervous system lesion).
Osseous Types.
Occipitocervical synostosis, basilar impression, and odontoid anomalies are the most common congenital/developmental malformations of the occipitovertebral junction, with an incidence of 1.4 to 2.5 per 100 children (50). These lesions arise from a malformation of the mesenchymal anlages at the occipitovertebral junction.
Basilar Impression.
Basilar impression is an indentation of the skull floor by the upper cervical spine. The tip of the dens is more cephalad and sometimes protrudes into the opening of the foramen magnum. This may encroach on the brain stem, risking neurologic damage from direct injury, vascular compromise, or alterations in cerebrospinal fluid flow (51).
Basilar impression can be primary or secondary. Primary basilar impression, the most common type, is a congenital abnormality often associated with other vertebral defects (e.g., Klippel-Feil syndrome, odontoid abnormalities, atlantooccipital fusion, and atlas hypoplasia). The incidence of primary basilar impression in the general population is 1% (52).
Secondary basilar impression is a developmental condition attributed to softening of the osseous structures at the base of the skull. Any disorder of osseous softening can lead to secondary basilar impression. These include metabolic bone diseases [e.g., Paget disease (53), renal osteodystrophy, rickets, osteomalacia (54, 55)] bone dysplasias and mesenchymal syndromes [e.g., osteogenesis imperfecta (56, 57, 58, 59, 60, 61 and 62), achondroplasia (63), hypochondroplasia (64), neurofibromatosis (65), and rheumatologic disorders, e.g., rheumatoid arthritis, ankylosing spondylitis]. The softening allows the odontoid to migrate cephalad and into the foramen magnum.
These patients typically present with a short neck (78% in one series) (66). This shortening is only an apparent deformity because of the basilar impression. Asymmetry of the skull and face (68%), painful cervical motion (53%), and torticollis (15%) can also occur. Neurologic signs and symptoms are often present (67). Many children will have acute onset of symptoms precipitated by minor trauma (68). In cases of isolated basilar impression, the neurologic involvement is primarily a pyramidal syndrome associated with proprioceptive sensory disturbances (motor weakness, 85%; limb paresthesias, 85%). In cases of basilar impression associated with Arnold-Chiari malformations, the neurologic involvement is usually cerebellar, and symptoms include motor incoordination with ataxia, dizziness, and nystagmus. In both types, the patients may complain of neck pain and headache in the distribution of the greater occipital nerve and cranial nerve involvement, particularly those that emerge from the medulla oblongata (trigeminal [V], glossopharyngeal [IX], vagus [X], and hypoglossal [XII]). Ataxia is a very common finding in children with basilar impression (68). Hydrocephalus may develop as a result of obstruction of the cerebrospinal fluid flow by obstruction of the foramen magnum from the odontoid.
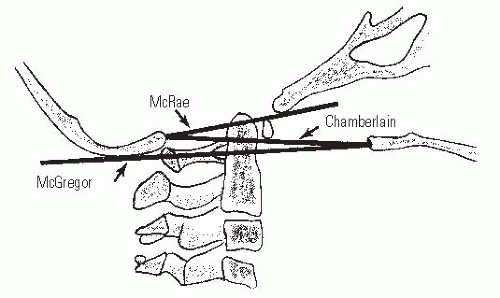
Basilar impression is difficult to assess radiographically. The most commonly used lines are Chamberlain (69), McRae (70), and McGregor (71) (Fig. 21-7). McGregor line is the best line for screening because the landmarks can be clearly defined at all ages on a routine lateral radiograph. McRae line is helpful in assessing the clinical significance of basilar impression because it defines the opening of the foramen magnum; in patients who are symptomatic, the odontoid projects above this line. At present, CT with sagittal plane reconstructions can show the osseous relations at the occipitocervical junction more clearly, and magnetic resonance imaging (MRI) clearly delineates the neural anatomy. MRI and CT norms have been recently established (72, 73). Occasionally, vertebral angiography or MR angiogram (MRA) is needed (74).
Treatment of basilar impression can be difficult and requires a multidisciplinary approach (orthopaedic, neurosurgical, and neuroradiologic) (59, 61, 75, 76, 77 and 78). The symptoms rarely can be relieved with customized orthoses (79); the primary treatment is surgical. If the symptoms are caused by a hypermobile odontoid, surgical stabilization in extension at the occipitocervical junction is needed. Anterior excision of the odontoid is needed if it cannot be reduced (80), but this should be preceded by posterior stabilization and fusion. In some cases, the anterior decompression can be performed endoscopically (81). If the symptoms are from posterior impingement, suboccipital decompression and often upper cervical laminectomy are needed. The dura often needs to be opened to look for a tight posterior band (66, 82). Posterior stabilization also should be performed (83). In a recent series of 190 cases, decompression of the foramen magnum was appropriate for those without an
Arnold-Chiari malformation; transoral anterior decompression was reserved for those with an associated Arnold-Chiari malformation (84). These are general statements, and each case must be considered individually. Secondary basilar impression tends to progress despite arthrodesis (61).
Arnold-Chiari malformation; transoral anterior decompression was reserved for those with an associated Arnold-Chiari malformation (84). These are general statements, and each case must be considered individually. Secondary basilar impression tends to progress despite arthrodesis (61).
Atlantooccipital Anomalies.
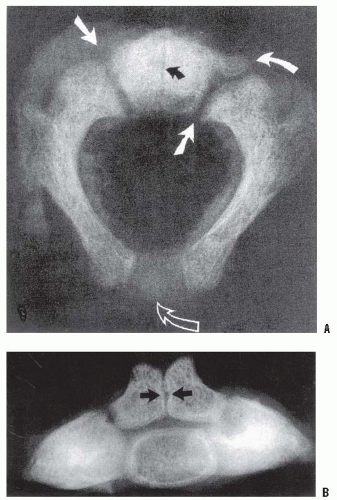
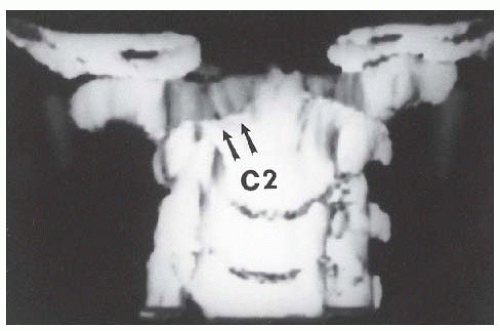
Children with congenital bony anomalies of the atlantooccipital junction present with a wide spectrum of deformities (85). The anterior arch of C1 is commonly assimilated to the occiput, usually in association with a hypoplastic ring posteriorly (Fig. 21-8), as well as condylar hypoplasia (86). The height of C1 is variably decreased, allowing the odontoid to project upward into the foramen magnum (i.e., primary basilar impression). More distal cervical anomalies can also occur in association with the atlantooccipital anomaly. The odontoid may be misshapen or directed posteriorly more than normal. Up to 70% of children with this condition have a congenital fusion of C2-C3 (see Fig. 21-8). (Posterior congenital fusion of C2-C3 is a clue that occiput-C1 anomalies, or other more distal cervical fusions, may be present. These may be cartilaginous initially, and not appear on plain radiographs until the child becomes more mature.) The fusion of C1 to the occiput is classified by zones (86); zone 1 is a fused anterior arch, zone 2 a fusion of the lateral masses, zone 3 a fused posterior arch, and zone 4 a combination of zones. Zone 4 fusions are most common, but the highest prevalence of spinal canal encroachment is in zone 2 patients.
Clinically, these children resemble those with the Klippel-Feil syndrome: short, broad necks; restricted neck motion; low hairline; high scapula; and torticollis (Fig. 21-9) (82, 87, 88 and 89). Recently, hemifacial microsomia has been noted to have associated atlantooccipital anomalies (90), as well as children with the 22q11.2 deletion syndrome (91). The skull may demonstrate a positional deformational plagiocephaly. They also may have other associated anomalies, including dwarfism, funnel chest, jaw anomalies, cleft palate, congenital ear deformities, hypospadias, genitourinary tract defects, and syndactyly. They can present with neurologic symptoms during childhood, but more often present between 40 to 50 years of age. These symptoms can be initiated by traumatic or inflammatory processes, and they progress slowly and relentlessly. Rarely do they present suddenly or dramatically, although they have been reported as a cause of sudden death. The most common signs and symptoms, in decreasing order of frequency, are neck and occipital pain, vertigo, ataxia, limb paresis, paresthesias, speech disturbances, hoarseness, diplopia, syncope, auditory malfunction, and dysphagia (92, 93).
Standard radiographs are difficult to obtain because of fixed bony deformities and overlapping shadows from the mandible, occiput, and foramen magnum. An x-ray beam directed 90 degrees perpendicular to the skull (rather than the cervical spine) usually gives a satisfactory view of the occipitocervical junction. The anomaly usually is studied further with CT. In young children, the head-wag autotomography technique can be quite useful (94). This technique involves side-to-side rotation of the child’s head, while a slow AP radiographic exposure of the upper cervical spine is performed. This rotation blurs the overlying head and mandibular structures, allowing for improved visualization of the occiput-C1-C2 complex.
The position of the odontoid relative to the opening of the foramen magnum has been described by measuring the distance from the posterior aspect of the odontoid to the posterior ring of C1 or the posterior lip of the foramen magnum, whichever is closer (87, 95). This should be determined in flexion because this position maximizes the reduction in the SAC. If this distance is <19 mm, a neurologic deficit is usually present. Lateral flexion and extension views of the upper cervical spine often show up to 12 mm of space between the odontoid and the C1 ring anteriorly (87); associated C1-C2 instability has been reported to develop eventually in 50% of these patients.
MRI is used to image the neural structures. Flexion-extension MRI is often necessary to fully evaluate the pathology (96). Compression of the brain stem or upper cervical cord anteriorly occurs from the backward-projecting odontoid. This produces a range of findings and symptoms, depending on the location and degree of compression. Pyramidal tract signs and symptoms (e.g., spasticity, hyperreflexia, muscle weakness, gait disturbances) are most common, although signs of cranial nerve involvement (e.g., diplopia, tinnitus, dysphagia, auditory disturbances) can be seen. Compression from the posterior lip of the foramen magnum or dural constricting band can disturb the posterior columns, with a loss of proprioception, vibration, and tactile senses. Nystagmus also occurs frequently as a result of posterior cerebellar compression. Vascular disturbances from vertebral artery involvement can result in brain stem ischemia, manifested by syncope, seizures, vertigo, and unsteady gait (97). Cerebellar tonsil herniation can occur. The altered mechanics of the cervical spine may result in a dull, aching pain in the posterior occiput and the neck with intermittent stiffness and torticollis. Irritation of the greater occipital nerve may cause tenderness in the posterior scalp.
The natural history of atlantooccipital anomalies is unknown. The neurologic symptoms may develop so late and progress so slowly because the frequently associated C1-C2 instability progresses with age, and the increased demands placed on the C1-C2 interval produce gradual spinal cord or vertebral artery compromise.
Treatment is difficult. Surgery for atlantooccipital anomalies is more risky than with isolated anomalies of the odontoid (82, 93). For this reason, nonoperative methods should be initially attempted. Cervical collars, braces, and traction often help for persistent complaints of head and neck pain, especially after minor trauma or infection. Immobilization may achieve only temporary relief if neurologic deficits are present. Patients with evidence of a compromised upper cervical area should take precautions not to expose themselves to undue trauma.
When symptoms and signs of C1-C2 instability are present, a posterior C1-C2 fusion is indicated. Preliminary traction to attempt reduction is used if necessary. If a reduction is possible and there are no neurologic signs, surgery has an improved prognosis (82, 92, 93). Posterior signs and symptoms may be an indication for posterior decompression depending on the evidence of dural or osseous compression. Results vary from complete resolution to increased deficits and death (82, 98). In the instance of no instability but only compressive pathology, the role of concomitant posterior fusion has not yet been determined. However, if decompression, whether anterior or posterior, can destabilize the spine, then concomitant posterior fusion should be considered (86, 99, 100).
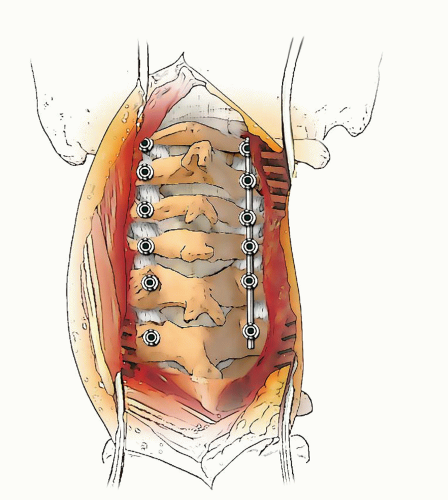
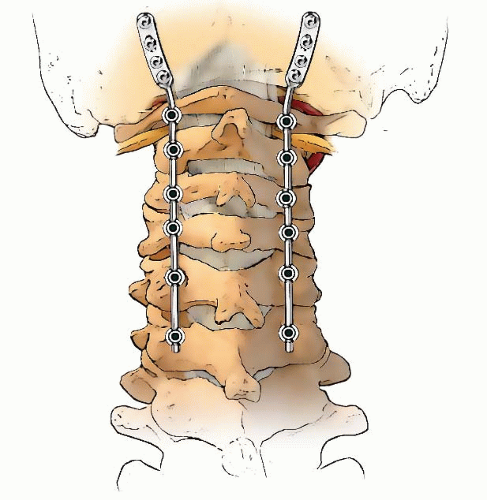
If occiput-C1 instability is present, then occiput-atlantocervical fusion is indicated (Figs. 21-10, 21-11, 21-12, 21-13, 21-14, 21-15 and 21-16). These cases are often associated with posterior decompressions/laminectomies.
Occipitoatlantocervical instability requiring occipitocervical fusion is often complicated by the fact that decompression of the base of the skull and the upper two cervical vertebrae is either required or the cause of the instability. These circumstances compromise the ability to achieve fusion because of the lack of bone surface to form a bed for the grafts, the large gap that must be bridged, and the instability (101). In addition, it has been demonstrated that the conventional cervical orthoses do not provide much immobilization for the upper cervical spine (102).
To circumvent these problems and to provide stability to the fusion area, surgeons have developed methods of internal fixation (103, 104). Since these reports, fixation using screws and plates has become more popular in many centers, but these techniques require a considerable learning curve and are often not amenable to the very small child with small anatomy or the dysplastic anatomy secondary to dysplasia/dwarfisms. In the small child, a useful technique that has proved effective is the use of rib or other cortical-cancellous graft to provide an element of stability, as well as to serve as the graft for the fusion. This is supplemented by the use of a halo orthosis. A combined team approach with neurosurgery is often helpful in these complicated cases.
Unilateral Absence of C1.
This congenital malformation of the first cervical vertebra is, in essence, a hemiatlas or a congenital scoliosis of C1. Doubousset (105) described 17 patients with this absence. No definite population incidence is known. The problem often is associated with other anomalies common to children with congenital spine deformities (e.g., tracheoesophageal fistula).
Two-thirds of the children present at birth; the others develop torticollis and are noticed later. A lateral translation of the head on the trunk, with variable degrees of lateral tilt and rotation (best appreciated from the back) is the typical finding. There also may be severe tilting of the eye line. The sternocleidomastoid muscle is not tight, although regional aplasia of the muscles in the nuchal concavity of the tilted side is noted. Neck flexibility is variable and decreases with age. The condition is not painful. Plagiocephaly can occur and increases as the deformity increases. Neurologic signs (e.g., headache, vertigo, myelopathy) are present in about one-fourth of the patients. The natural history is unknown.
Occipitocervical Facet Fusion After Laminectomy (Figs. 21-10, 21-11, 21-12, 21-13, 21-14, 21-15 and 21-16).
Standard AP and lateral radiographs rarely give the diagnosis, although the open-mouth odontoid view may suggest it. Tomograms or CT scans usually are needed to see the anomaly (Fig. 21-17). The defect can range from a hypoplasia of the lateral mass to a complete hemiatlas with rotational instability and basilar impression. Occasionally, the atlas is occipitalized. There are three types of this disorder. Type I is an isolated hemiatlas. Type II is a partial or complete aplasia of one hemiatlas, with other associated anomalies of the cervical spine (e.g., fusion of C3-C4, congenital bars in the lower cervical vertebrae). Type III is a partial or complete atlantooccipital fusion and symmetric or asymmetric hemiatlas aplasia, with or without anomalies of the odontoid and the lower cervical vertebrae.
Once this malformation is diagnosed, entire spinal radiographs should be taken to rule out other congenital vertebral anomalies. Other imaging studies that may be needed are vertebral angiography and MRI. Angiography should be performed if operative intervention is undertaken, because arterial anomalies (e.g., multiple loops, vessels smaller than normal, abnormal routes between C1 and C2) often are found on the aplastic side. MRI also should be performed if operative intervention is undertaken, because many of these children will have stenosis of the foramen magnum, and occasionally an Arnold-Chiari malformation.
The deformity should be observed to document the presence or absence of progression. This observation is primarily clinical (e.g., photographs) because radiographic measurements are difficult if not impossible to obtain. Bracing does not halt progression of the deformity. Surgical intervention is recommended in those patients with severe deformities. A preoperative halo is used for gradual traction correction over 6 to 8 days. An ambulatory method of gradual cervical spine deformity correction has been described using the halo-Ilizarov technique (106). A posterior fusion from the occiput-C2 or -C3 is then performed, depending on the extent of the anomaly. Decompression of the spinal canal is necessary when the canal size is not ample, either at that time or if it is projected not to be able to fully accommodate the developed spinal cord. The ideal age for posterior fusion is between the ages of 5 and 8 years, corresponding to the age at which the canal size reaches adult proportions.
Familial Cervical Dysplasia.
The epidemiology of this atlas deformity (107) is not known. Clinical presentation varies from an incidental finding to a passively correctable head tilt,
suboccipital pain, decreased cervical motion, or a clunking of the upper cervical spine.
suboccipital pain, decreased cervical motion, or a clunking of the upper cervical spine.
Plain radiographs are difficult to interpret. Various anomalies of C1, most commonly a partial absence of the posterior ring of C1, typically are seen. Various anomalies of C2 also commonly exist, for example, a shallow hypoplastic left facet. Other dysplasias of the lateral masses, facets, and posterior elements and occasionally spondylolisthesis are seen. Occiput-C1 instability is frequently seen; C1-C2 instability rarely is seen. The delineation of this complex anatomy often is seen best with a CT scan and a three-dimensional reconstruction (Fig. 21-18). When symptoms of instability are present, MRI in flexion and extension is recommended to assess the presence and magnitude of neural compression. Occipitocervical junction instability due to the malformation may lead to neural compromise.
Nonsurgical treatment consists of observation every 6 to 12 months to ensure that instability does not develop, either clinically (e.g., progressive weakness and fatigue or objective signs of myelopathy), or radiographically, with lateral flexion and extension radiographs. Surgical intervention is recommended for persistent pain, torticollis, and neurologic symptoms. A posterior fusion from the occiput to C2 usually is required, with gradual preoperative reduction using an adjustable halo cast (106).
Atlantoaxial Rotary Displacement.
Atlantoaxial rotary displacement is one of the most common causes of childhood torticollis. Rotary displacements are characteristically a pediatric problem, but they may occur in adults. There are several causes. Because the resultant radiographic findings and treatment regimens are the same for all pediatric causes, they are discussed as a unit and individual exceptions are noted where necessary.
The confusing terminology includes rotary dislocation, rotary deformity, rotational subluxation, rotary fixation, and spontaneous hyperemic dislocation (108, 109). “Atlantoaxial rotary subluxation” is probably the most accepted term used in describing the common childhood torticollis. “Subluxation” is misleading, however, because cases of “subluxation” usually present within the normal range of motion of the atlantoaxial joint. “Rotary displacement” is a more appropriate and descriptive term because it includes the entire range of pathology, from mild subluxation to complete dislocation. If the deformity persists, the children present with a resistant and unresolving torticollis that is best termed “atlantoaxial rotary fixation or fixed atlantoaxial displacement.” Gradations exist between the very mild, easily correctable rotary displacement and the rigid fixation. Complete atlantoaxial rotary dislocation rarely has been reported in surviving patients.
The radiographic findings of rotary displacement are difficult to demonstrate (110). With rotary torticollis the lateral mass of C1 that has rotated anterior appears wider and closer to the midline (medial offset), whereas the opposite lateral mass is narrower and away from the midline (lateral offset). The facet joints may be obscured because of apparent overlapping. The lateral view shows the wedge-shaped lateral mass of the atlas lying anteriorly where the oval arch of the atlas normally lies, and the posterior arches fail to superimpose because of the head tilt (Fig. 21-19). These findings may suggest occipitalization of C1 because with the neck tilt the skull may obscure C1. The normal relation between the occiput and C1 is believed to be maintained in children with atlantoaxial rotary displacement. A lateral radiograph of the skull may demonstrate the relative positions of C1 and C2 more clearly than a lateral radiograph of the cervical spine. This is because tilting of the head also tilts C1, which creates overlapping shadows and makes interpretation of a lateral spinal radiograph difficult.
The difficulty with plain radiographs is differentiating the position of C1-C2 in a child with subluxation from that in a normal child whose head is rotated, since both give the same picture. Open-mouth views are difficult to obtain and interpret, and the lack of cooperation and the diminished motion on the part of the child often make it impossible to obtain these special views. Cineradiography has been recommended, but the radiation dose is high and patient cooperation may be difficult because of muscle spasms (110, 111). CT scans are helpful in this situation if it is done properly (112). A CT scan, when taken with the head in the torticollic position, may be interpreted by the casual observer as showing rotation of C1 on C2. If the rotation of C1 on C2 is within the normal range, as it usually is early on in this condition, the observer may attribute this rotation to patient positioning. A dynamic-rotation CT scan is helpful here. Views with the head maximally rotated to the right, and then to the left, will demonstrate atlantoaxial rotary fixation when there is a loss of normal rotation (Fig. 21-19). There are varying degrees of “locking” between the C1 and C2 vertebrae (113, 114 and 115).
Rotary displacement can be classified into four types (Fig. 21-20) (108): type I is a simple rotary displacement without an anterior shift, type II is rotary displacement
with an anterior shift of 5 mm or less, type III is rotary displacement with an anterior shift >5 mm, and type IV is rotary displacement with a posterior shift. The amount of anterior displacement considered to be pathologic is >3 mm in older children and adults and >4 mm in younger children (33). Flexion and extension lateral-stress radiographs are suggested to rule out the possibility of anterior displacement.
with an anterior shift of 5 mm or less, type III is rotary displacement with an anterior shift >5 mm, and type IV is rotary displacement with a posterior shift. The amount of anterior displacement considered to be pathologic is >3 mm in older children and adults and >4 mm in younger children (33). Flexion and extension lateral-stress radiographs are suggested to rule out the possibility of anterior displacement.
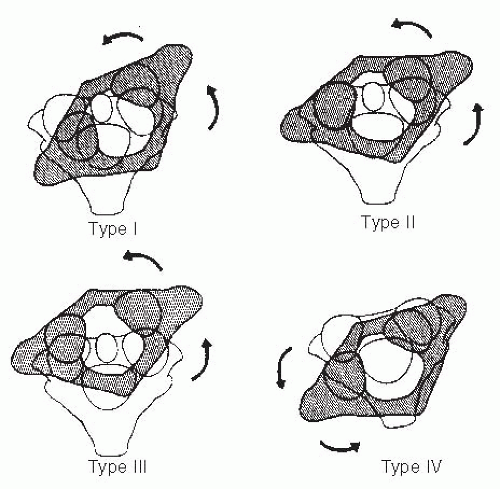 FIGURE 21-20. The four types of atlantoaxial rotary displacement. (From Fielding JW, Hawkins RJ. Atlanto-axial rotatory fixation. J Bone Joint Surg Am 1977;59-A:37-44, with permission.) |
Type I is the most common pediatric type. It is usually benign and frequently resolves by itself. Type II deformity is potentially more dangerous. Types III and IV are very rare, but because of the potential for neurologic involvement and even instant death, management must be approached with great caution.
The etiology and pathoanatomy is not known completely (116). Several causative mechanisms are possible. Cervical spine fracture is a rare etiology. More commonly, atlantoaxial rotary displacement occurs following minor trauma [e.g., clavicle fractures (117)], after head and neck surgery including simple central line insertion (118), or after an upper respiratory infection. The children present with a “cocked-robin” torticollis and resist any attempt to move the head because of pain. The associated muscle spasm is noted on
the side of the long sternocleidomastoid muscle because the muscle is attempting to correct the deformity, unlike congenital muscular torticollis where the muscle causes the torticollis. If the deformity becomes fixed, the pain subsides but the torticollis persists, along with decreased neck motion. In longstanding cases, plagiocephaly and facial flattening may develop on the side of the tilt.
the side of the long sternocleidomastoid muscle because the muscle is attempting to correct the deformity, unlike congenital muscular torticollis where the muscle causes the torticollis. If the deformity becomes fixed, the pain subsides but the torticollis persists, along with decreased neck motion. In longstanding cases, plagiocephaly and facial flattening may develop on the side of the tilt.
Spontaneous atlantoaxial subluxation with inflammation of adjacent neck tissues, also known as Grisel syndrome, is commonly seen in children after upper respiratory infections (Fig. 21-21). The children are frequently febrile (119). A direct connection exists between the pharyngovertebral veins and the periodontal venous plexus and suboccipital epidural sinuses (120). This may provide a route for hematogenous transport of peripharyngeal septic exudates to the upper cervical spine and an anatomic explanation for the atlantoaxial hyperemia of Grisel syndrome. In longstanding cases, soft-tissue abscesses or vertebral osteomyelitis may develop (121, 122 and 123). Regional lymphadenitis is known to cause spastic contracture of the cervical muscles. This muscular spasm, in the presence of abnormally loose ligaments (hypothetically caused by the hyperemia of the pharyngovertebral vein drainage), could produce locking of the overlapping lateral joint edges of the articular facets. This prevents easy repositioning, resulting in atlantoaxial rotary displacement. The hyperemia after surgery of the oral pharynx, most frequently tonsillectomy and adenoidectomy, enhances the passage of the inflammatory products into the pharyngovertebral veins. It is known that patients may develop Grisel syndrome after otolaryngologic procedures (124), especially with monopolar electrocautery (125). Kawabe and Tang (126, 127) have demonstrated meniscuslike synovial folds in the atlantooccipital and lateral atlantoaxial joints of children, but not in those of adults, and have found that the dens-facet angle of the axis is steeper in children than in adults. They postulate that excessive C1-C2 rotation, caused by the steeper angle, compounded by ligament laxity from an underlying hyperemia, allows the meniscus-like synovial folds to become impinged in the lateral atlantoaxial joint, leading to rotary fixation. The predominance of this syndrome in childhood correlates with the predilection for the adenoids to be maximally hypertrophied and inflamed at this same time, and located in the area drained by the pharyngo-vertebral veins.
Most atlantoaxial rotary displacements resolve spontaneously. Rarely, however, the pain subsides and the torticollis becomes fixed. The duration of symptoms and deformity dictates the recommended treatment (128).
Patients with rotary subluxation of <1 week can be treated with immobilization in a soft cervical collar and rest for about 1 week. Close follow-up is mandatory. If spontaneous reduction does not occur with this initial treatment, hospitalization and the use of halter traction, muscle relaxants (e.g., diazepam), and analgesics is recommended next. Patients with rotary subluxation of >1 week but <1 month should be hospitalized immediately for cervical traction, relaxants, and analgesics. Gentle halo traction is occasionally needed to achieve reduction. The reduction is noted clinically and confirmed with a dynamic CT scan. If no anterior displacement is noted after reduction, cervical support should be continued only as long as symptoms persist. If there is anterior displacement, immobilization should be continued for 6 weeks to allow ligamentous healing to occur. In patients with rotary subluxation for more than 1 month, cervical traction (usually halo skeletal) can be tried for up to 3 weeks, but the prognosis is guarded. These children usually fall into two groups: those whose rotary subluxation can be reduced with halo traction but, despite a prolonged period of immobilization, resubluxate when the immobilization is stopped and those whose subluxation cannot be reduced, and is fixed. It has been recently shown that those patients with recurrence of deformity have a larger difference in the lateral mass-dens interval on the initial AP radiograph (129).
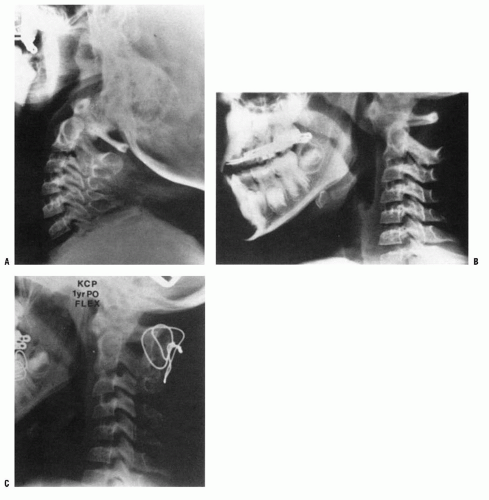
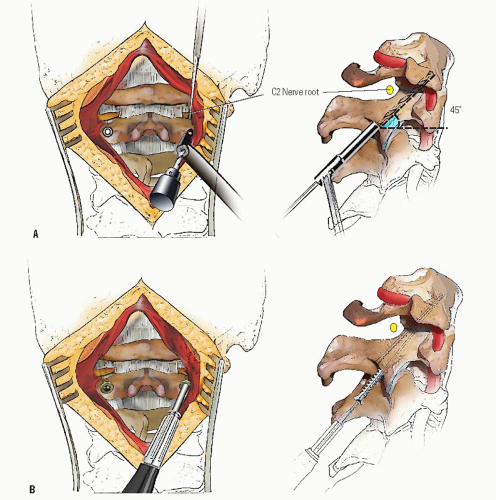
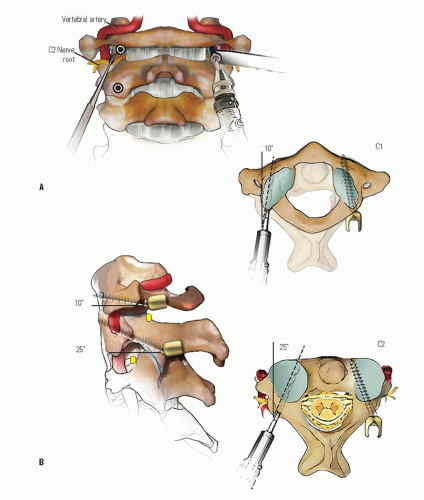
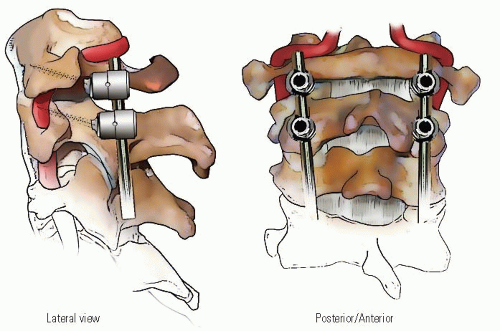
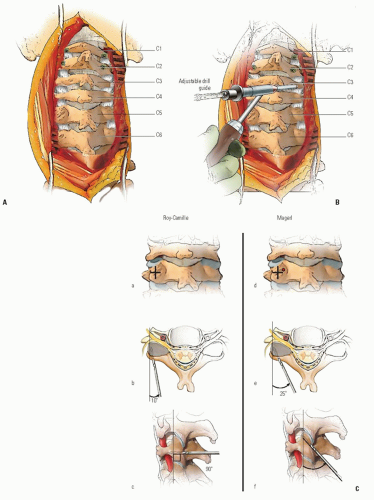
When the deformity is fixed, especially when anterior C1 displacement is present, the transverse atlantal ligament is compromised with a potential for catastrophe. In this situation, posterior C1-C2 fusion should be performed. The indications for fusion are neurologic involvement, anterior displacement, failure to achieve and maintain correction, a deformity that has been present for more than 3 months, and recurrence of deformity following an adequate trial of conservative management (at least 6 weeks of immobilization after reduction). Before surgical fusion halo traction for several days is used to obtain as much straightening of the head and neck as possible, a forceful or manipulative reduction should not be performed. Postoperatively, the child is simply positioned in a halo cast or vest in the straightened position obtained preoperatively; this usually obtains satisfactory alignment. A Gallietype fusion with sublaminar wiring at the ring of C1 and through the spinous process of C2 is preferred to a Brookstype fusion in which the wire is sublaminar at both C1 and C2. This is because of the decreased SAC at C2 with a higher risk of neurologic injury. This wiring does not reduce the displacement but simply provides some internal stability for the arthrodesis. The overall results for a Gallie fusion are very good (see Figs. 21-22, 21-23, 21-24, 21-25, 21-26, 21-27, 21-28, 21-29 and 21-30) (131). Long-term results do not indicate any significant abnormalities of the sagittal profile (134). In the nonreduced rotatory fixation, transarticular C1-C2 screw fixation is contraindicated due to the pathologic anatomy from the rotational deformity (Figs. 21-31, 21-32, 21-33, 21-34, 21-35, 21-36 and 21-37). A few surgeons advocate reduction of the deformity (135, 136); if a fusion is later needed and the deformity reduced, then transarticular C1-C2 screw fixation can be used if appropriate size screws are available.
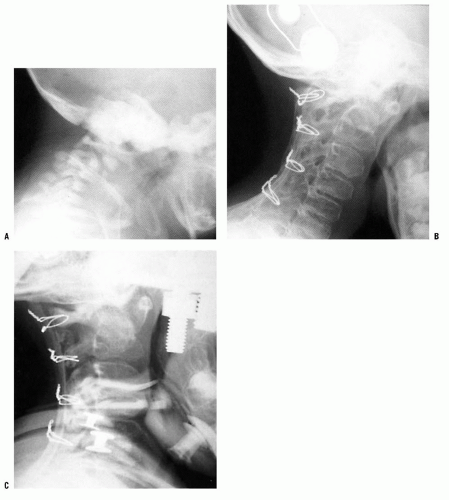
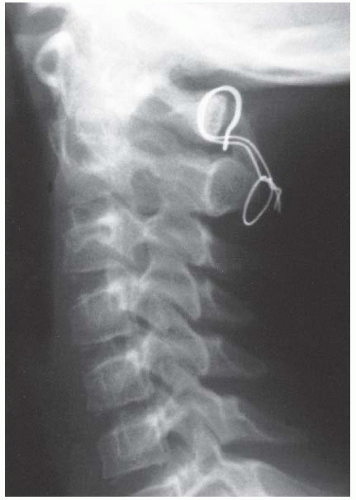 FIGURE 21-22. The child in Figure 21-19 had a fixed deformity that occurred 6 months earlier, immediately after reconstructive maxillofacial surgery for Goldenhar syndrome. It did not respond to traction, including halo traction. She underwent a posterior C1-C2 (Gallie-type) fusion. A solid fusion was present 9 months later; clinically, the patient achieved 80 degrees of rotation to the left and 45 degrees of rotation to the right. |
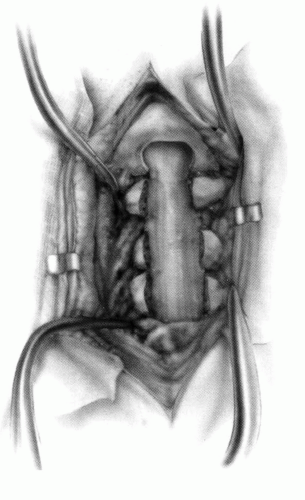
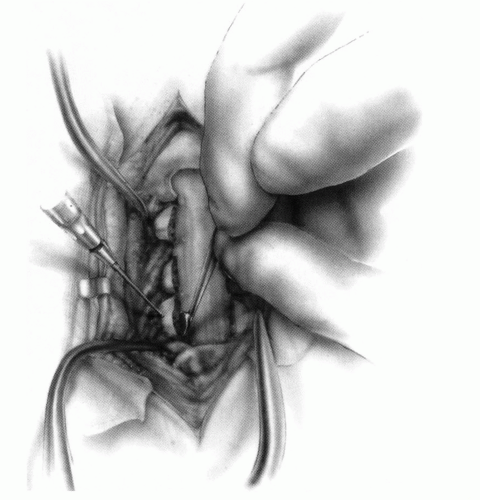
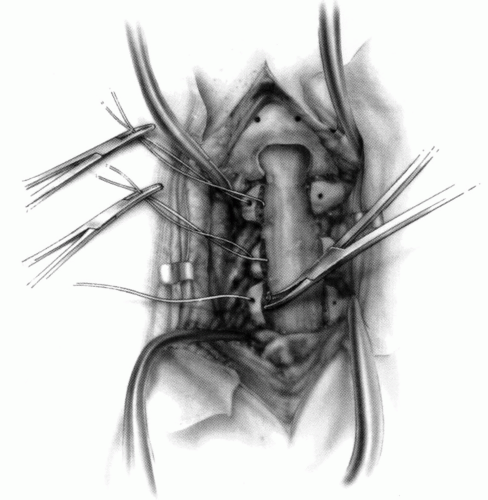
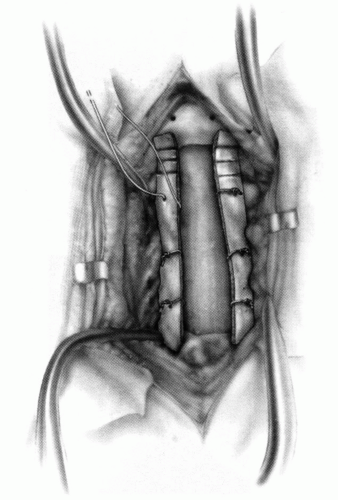
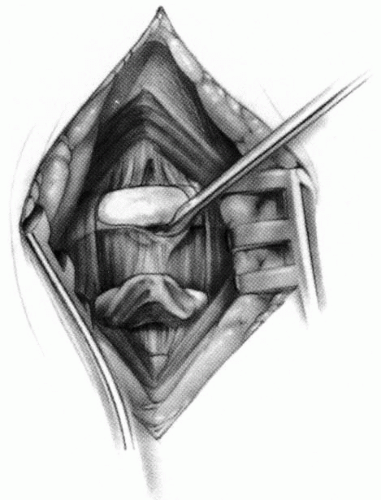
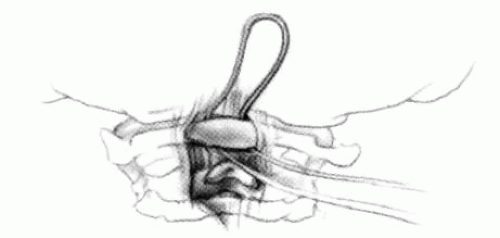
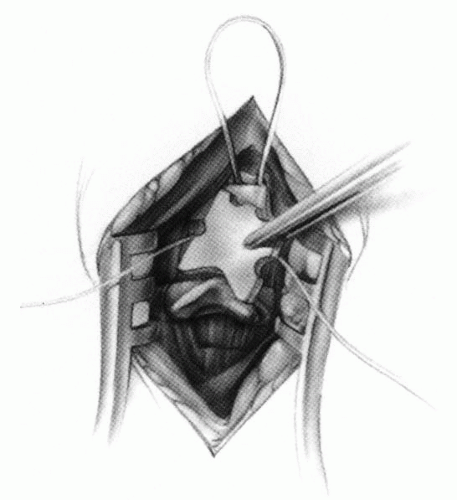
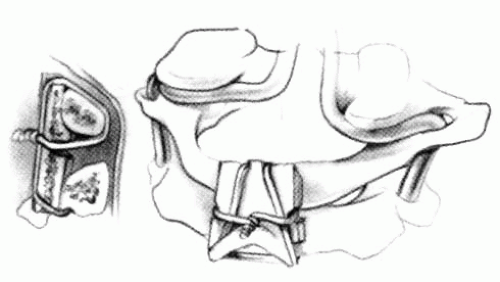
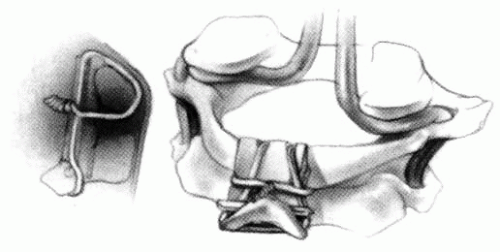
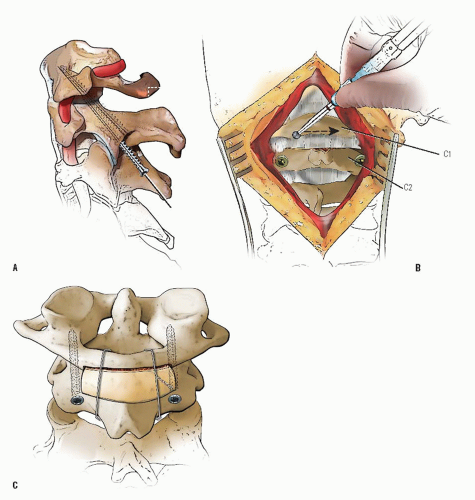
 FIGURE 21-23. The most common arthrodesis of the cervical spine is between the axis and the atlas because of the numerous congenital and developmental problems that affect this region. Although several techniques have been advocated to achieve arthrodesis of these vertebrae, the technique attributed to Gallie (130) is the most reliable and the easiest to apply in children. In this technique, the wire not only helps to pull C1 back into position and hold it there but also holds the bone graft firmly in place (131, 132). Occasionally, the posterior arch of C1 is not formed completely, making this technique impossible; in these cases other techniques need to be used, such as only grafting with halo immobilization (104). In cases in which there is a great deal of instability with chance for neurologic injury, it is preferred to place the patient in a halo vest or cast first. This can be done under local or general anesthesia, as needed. Reduction is achieved and confirmed by radiographs. If the halo was applied with the patient awake, anesthesia is then induced and the child turned prone for the posterior fusion. No head rest is necessary, and there is little danger of neurologic injury while carefully intubating and moving the patient with the halo vest in place. The occipital region of the skull is shaved, and the posterocervical area and the posterior iliac crest are prepared and draped. The incision extends in the midline from the base of the skull to the spinous process of C4. Dissection is carried down to the tips of the spinous processes. At this point, a metal hub needle is placed in the spinous process of C2 and a lateral radiograph is taken. This is done to positively identify the correct vertebrae for exposure. In the young child, exposure of the base of the skull or any additional vertebrae may result in “creeping fusion.” |
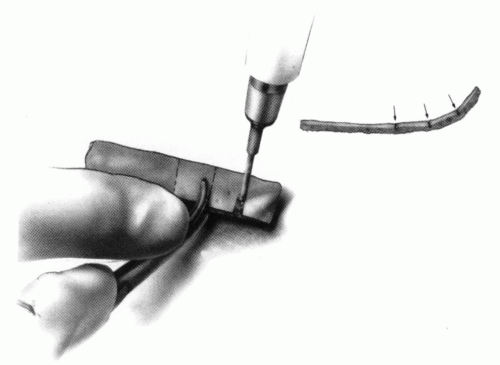
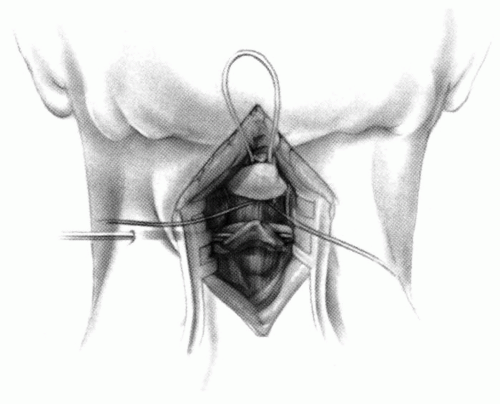 FIGURE 21-28. In children, the spinous process of C2 is often small and does not provide much strength for fixation of the wire. The spinous process K-wire technique is an alternative technique (133). A threaded K wire of appropriate size is passed through a small stab wound on the side of the neck and through the paravertebral muscles and is drilled through the spinous process of C2. It is cut so that approximately 1 cm is protruding on each side. |
Author’s Preferred Treatment.
Patients with rotary subluxation of <1 week are treated with immobilization in a soft cervical collar and rest for about 1 week. If spontaneous reduction does not occur, halter traction, muscle relaxants (e.g., diazepam), and analgesics are prescribed. Patients with rotary subluxation of >1 week but <1 month should be hospitalized immediately for cervical traction, relaxants, and analgesics. Gentle halo traction is occasionally needed to achieve reduction. All reductions are confirmed with a dynamic CT scan. In patients with rotary subluxation for more than 1 month, cervical traction (usually halo skeletal) can be tried for up to 3 weeks, but the prognosis is guarded. If reduction cannot be achieved or maintained, then posterior C1-C2 arthrodesis is recommended (Fig. 21-23).
Nonosseous Types
Congenital Muscular Torticollis.
Congenital muscular torticollis, or congenital wry neck, is the most common cause of torticollis in the infant and young child, presenting at a median age of 2 months (137). The incidence of soft-tissues abnormalities in the neck as documented by ultrasound was approximately 4% in a series of 1021 newborn infants (138). The deformity is caused by contracture of the sternocleidomastoid muscle, with the head tilted toward the involved side and the chin rotated toward the opposite shoulder. A disproportionate number of these children have a history of a primiparous birth or a breech or difficult delivery. However, it has been reported in children with normal births and those born by cesarean section (137, 139, 140).
The exact cause is not known and there are several theories. Because of the birth history, one theory is that of a compartment syndrome occurring from soft-tissue compression of the neck at the time of delivery (141). Surgical histopathologic sections suggest venous occlusion of the sternocleidomastoid muscle (142). This occlusion may result in a compartment syndrome, as manifested by edema, degeneration of muscle fibers, and muscle fibrosis. This fibrosis is variable, ranging from small amounts to the entire muscle. It has been suggested that the clinical deformity is related to the ratio of fibrosis to remaining functional muscle. If ample muscle remains, the sternocleidomastoid will probably stretch with growth, and the child will not develop torticollis; if fibrosis predominates, there is little elastic potential, and torticollis will develop. Another theory is in utero crowding, since three of four children have the lesion on the right side (143) and up to 20% have developmental hip dysplasia (144). The fact that this condition can occur in children with normal birth histories or in children born by cesarean section challenges the perinatal compartment syndrome theory, and supports the in utero crowding theory. The fact that it can occur in families (145, 146 and 147) (supporting a genetic predisposition) also questions the compartment syndrome theory. A third theory is primarily neurogenic (148), supported by histopathologic evidence of denervation and reinnervation. The primary myopathy initially may be due to trauma, ischemia, or both, and unequally involves the two heads of the sternocleidomastoid muscle. With continuing fibrosis of the sternal head, the branch of the spinal accessory nerve to the clavicular head of the muscle can be entrapped, leading to a later progressive deformity (148).
The final theory concerns mesenchymal cells remaining in the sternocleidomastoid from fetal embryogenesis. Recent histopathologic studies have demonstrated the presence of both myoblasts and fibroblasts in the sternocleidomastoid tumor in varying stages of differentiation and degeneration (149). The source of these myoblasts and fibroblasts is unknown. After birth, environmental changes stimulate these cells to differentiate, and the sternocleidomastoid tumor develops. Hemorrhagic and inflammatory reactions would be expected if the tumor was a result of perinatal birth trauma or intrauterine positioning, yet these cells were not seen in the sternocleidomastoid histopathologic studies. The occurrence of torticollis depends on the fate of the myoblasts in the mass. If the myoblasts undergo normal development and differentiation, then no persistent torticollis will occur and conservative treatment will likely succeed. If the myoblasts mainly undergo degeneration, then the remaining fibroblasts produce large amounts of collagen, with a scarlike contraction of the sternocleidomastoid muscle and the typical torticollis.
There are three clinical subgroups; those with sternocleidomastoid tumor (43% of cases), those with muscular torticollis (31% of cases), and postural torticollis (22% of cases) (150). The clinical features of congenital muscular torticollis depend on the age of the child. It is often discovered in the first 6 to 8 weeks of life. If the child is examined during the first 4 weeks of life, a mass or a “tumor” may be palpable in the neck (139). Although the mass may be palpable, it is unrecognized up to 80% of the time (151). Characteristically, it is a nontender, soft enlargement beneath the skin, and is located within the sternocleidomastoid muscle belly. This tumor reaches its maximum size within the first 4 weeks of life then gradually regresses. After 4 to 6 months of life, the contracture and the torticollis are the only clinical findings. In some children, the deformity is not noticed until after 1 year of age, which raises questions about both the congenital nature of this entity and the perinatal compartment syndrome theory. Recent studies (152) indicate that the rate of associated hip dysplasia in children with congenital muscular torticollis is 8%, lower than the previously cited 20% (144). The sternocleidomastoid tumor subgroup, the most severe group, presents at an earlier age, is associated with a higher incidence of breech presentation (19%), difficult labor (56%), and hip dysplasia (6.8%) (150).
If the deformity is progressive, skull and face deformities can develop (plagiocephaly) (153, 154), often within the first year of life. The facial flattening occurs on the side of the contracted muscle and is probably caused by the sleeping position of the child (155). In the United States, children usually sleep prone, and in this position, it is more comfortable for them to lie with the affected side down. The face therefore remodels to conform to the bed. If the child sleeps supine, reverse modeling of the contralateral skull occurs. In the child who is untreated for many years, the level of the eyes and ears becomes unequal and can result in considerable cosmetic deformity.
Ultrasound can be quite helpful in differentiating congenital muscular torticollis from other pathologies in the
neck (138, 156). Radiographs of the cervical spine should be obtained to rule out associated congenital anomalies. Plain radiographs of the cervical spine in children with muscular torticollis are always normal, aside from the head tilt and rotation. If any suspicion exists about the status of the hips, appropriate imaging (e.g., ultrasonography, radiography) should be done, depending on the age of the child and the expertise of the ultrasonographer.
neck (138, 156). Radiographs of the cervical spine should be obtained to rule out associated congenital anomalies. Plain radiographs of the cervical spine in children with muscular torticollis are always normal, aside from the head tilt and rotation. If any suspicion exists about the status of the hips, appropriate imaging (e.g., ultrasonography, radiography) should be done, depending on the age of the child and the expertise of the ultrasonographer.
Research MRI studies demonstrate abnormal signals in the sternocleidomastoid muscle, but no discrete masses within the muscle (141, 157). The muscle diameter is increased two to four times that of the contralateral muscle. In older patients the signals are consistent with atrophy and fibrosis, similar to those encountered in compartment syndromes of the leg and forearm.
As the deficit in cervical rotation increases, the incidence of a previous sternocleidomastoid tumor, hip dysplasia, and the likelihood of needing surgery increases (158, 159). Treatment initially consists of conservative measures (139, 140, 151, 160, 161). Good results can be expected with stretching exercises alone, with one series reporting 90% success (160) and another 95% (159). Those children with a sternocleidomastoid tumor respond less favorably to conservative stretching exercises than those with a simple muscle torticollis; none of the children with postural torticollis need surgery (159). The extent of sternocleidomastoid fibrosis on ultrasound examination is also predictive of the need for surgery (162, 163). In those cases in which only the lower one-third of the muscle is involved with fibrosis, all responded to conservative therapy, and in those cases where the entire length of the muscle was involved with fibrosis, surgery was needed in 35% of the children (164).
The exercises are performed by the caregivers and guided by the physiotherapist. The ear opposite the contracted muscle should be positioned to the shoulder, and the chin should be positioned to touch the shoulder on the same side as the contracted muscle. When adequate stretching has occurred in the neutral position, the exercises should be graduated up to the extended position, which achieves maximum stretching and prevents residual contractures. Treatment measures to be used along with stretching consist of room modifications by modifying the child’s toys and crib so that the neck is stretched when the infant is reaching for or looking at objects of interest. The exact role of the efficacy of these stretching measures, versus a natural history of spontaneous resolution, is not known (165); there are many anecdotal cases of spontaneous resolution. Occasionally, muscle stretching itself will result in partial or complete rupture of the sternocleidomastoid muscle (166). Recently, some have used botulinum toxin as an adjunct to assist in the stretching program, although dysphagia and neck weakness are significant side effects (167, 168 and 169).
If stretching measures are unsuccessful after 1 year of age (159, 161, 165, 170), surgery is recommended. The child’s neck and anatomic structures are larger, making surgery easier. Established facial deformity or a limitation of more than 30 degrees of motion usually precludes a good result, and surgery is required to prevent further facial flattening and further cosmetic deterioration (161). Asymmetry of the face and skull can improve as long as adequate growth potential remains after the deforming pull of the sternocleidomastoid is removed; good but not perfect results can be obtained as late as 12 years of life (151, 171, 172).
The best time for surgical release is between the ages of 1 and 4 years; (139, 173) for those treated surgically before the age of 3 years, excellent results can be expected in nearly all cases (171). Surgical treatments include a unipolar release (171) at the sternoclavicular or mastoid pole, bipolar release, middle third transection, and even complete resection. Although these surgical procedures are usually done open, endoscopic (174) and percutaneous (distal) approaches have been recently described (175). Bipolar release combined with a Z-plasty of the sternal attachment yielded 92% satisfactory results in one series, whereas only 15% satisfactory results were obtained with other procedures (170). Similar results, although not perfect, can even be achieved in older children with a bipolar release (172, 176). In a more recent series of surgical cases, excellent results were obtained with a unipolar release and aggressive postoperative stretching (171). Middle third transection has also been reported to give 90% satisfactory results (177). Z-plasty lengthening maintains the V-contour of the neck and cosmesis, which the middle third transection does not. Structures that can be injured from surgery are the spinal accessory nerve, the anterior and external jugular veins, the carotid vessels and sheath, and the facial nerve. Skin incisions should never be located directly over the clavicle because of cosmetically unacceptable scar spreading; rather, they should be made one finger breadth proximal to the medial end of the clavicle and sternal notch, and in line with the cervical skin creases. The postoperative protocol can vary from simple stretching exercises to cast immobilization. Some type of a bracing device to maintain alignment of the head and neck is probably a desirable part of the postoperative protocol (178).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree