Fig. 13.1
Advanced hemophilic elbow arthropathy in an adult hemophilic patient in our study who presented with intense joint pain
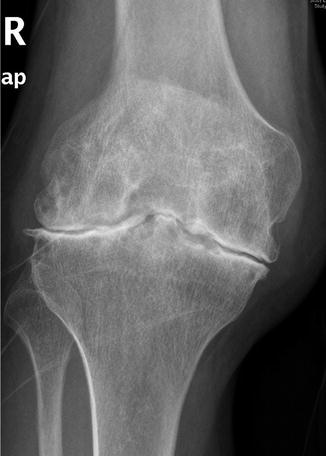
Fig. 13.2
Knee from an adult hemophilic patient in our series showing very advanced hemophilic arthropathy (AHA) associated with intense joint pain
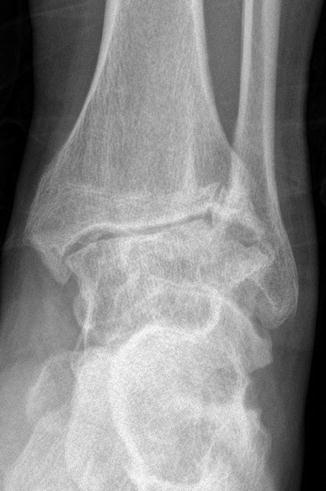
Fig. 13.3
Advanced hemophilic ankle arthropathy in an adult hemophilic patient in our study, who complained of intense joint pain
Table 13.1
Inclusion criteria for the study
Patients with severe hemophilia A (<1 % factor VIII) |
Age >18 years |
Advanced hemophilic arthropathy (AHA) as detected by radiology (over 10 points on Peterson’s scale) |
Joint pain >40 mm in elbows, knees, or ankles |
Absence of hemarthrosis and synovitis (as determined on ultrasonography) at the time of inclusion into the study |
Over a period of 2 months, patients in the control group (30 patients) were subjected to secondary hematologic prophylaxis (intravenous infusion of 3,000 IU of factor A/three times a week) combined with 650 mg/every 6 h of oral paracetamol (Gelocatil 60 mg, Laboratorios Gelos SL, Esplugues de Llobregat, Barcelona, Spain). The drugs were administered in 15-day cycles, with a 15-day resting period between cycles. Seventeen knees, nine elbows, and four ankles were treated. Mean patient age was 25.4 years (range: 21–50 years).
The study group (celecoxib) also included 30 patients, who were administered the same treatment as patients in the control group over 2 months, but with the addition of oral celecoxib (200 mg/once a day over 15 days followed by a 15-day resting period after which the cycle was repeated). Sixteen knees, eight elbows, and six ankles were treated. Mean patient age was 23.7 years (range: 20–58 years). Evaluation of pain was carried out by means of a visual analog scale (VAS, 0–100 mm, where 0 indicated no pain and 100 the worst imaginable pain) prior to treatment and at 2 months’ follow-up (treatment completion). Pain relief was considered poor when the treatment resulted in a decrease of less than 10 points on VAS, fair when the decrease was between 11 and 20 points, good when the reduction was between 21 and 40 points, and excellent when a decrease of between 41 and 60 points was obtained.
Descriptive and analytical statistics were used for data analysis. Results where p < 0.05 were considered significant. The SPSS 15.0 statistical package for Windows was used for the analysis.
Results of control group: the pretreatment pain score was 73.4 (range: 45–95). The posttreatment pain score was 37.3 (range: 25–35). Results in this group were excellent in 11 cases, good in six, fair in eight, and poor in five. No differences were observed between the different joints (elbows, knees, ankles) with respect to those results. No complications were reported in this group.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








