Severity
Factor levels (FVIII/FIX) (%)
Clinical pattern
Severe
<1
Severe. Spontaneous
Moderate
1–4
Moderate. Severe in surgery
Mild
5–25
Mild. After surgery
Joint hemorrhage (hemarthrosis), without a correct treatment, predisposes to recurrent bleeds, chronic synovitis, and hemophilic arthropathy [3]. The main goal of replacement therapy is to prevent the development of this pathology. On-demand treatment has been shown to slow the progression of arthropathy, but cannot prevent it [4]. The need for primary prophylaxis to prevent joint disease was established in the studies of Nilsson et al. in Sweden [5]. After 25-year follow-up, they observed that full-dose prophylaxis started in the first few years of life in boys with severe hemophilia prevented recurrent joint bleeds and preserved an excellent musculoskeletal status [5, 6]. They also observed that joint deterioration was frequent because of progressive destruction of joints already affected before the start of prophylaxis. This finding indicated that treatment should begin at a very early age, before joints are affected. This proposal for so-called primary prophylaxis is currently the emerging standard of care for patients with hemophilia without inhibitor around the world [7].
In the past four decades, hemophilia has changed from a fatal disease to a disorder with a safe and effective treatment available. The overall life expectancy of patients has dramatically increased in the last years [8]. Quality of life has also improved, mainly as a result of the different forms of prophylaxis. However, unfortunately, many adult patients still suffer from musculoskeletal complications such as chronic synovitis, fixed-joint flexion contractures, and hemophilic arthropathy. These complications result in the need for various surgical procedures such as arthrocentesis, synovectomies, and total joint arthroplasty [9]. Surgery in children is less common than in adults, because children do not have as many orthopedic complications. The most frequent surgical procedures in children with hemophilia are circumcision, dental procedures, insertion of a central venous access device (CVAD), and tonsillectomy [10].
2.2 Replacement Therapy for Orthopedic Procedures in Hemophilia
2.2.1 Optimal Level and Duration of Replacement Therapy
Surgery in patients with hemophilia carries a high risk of bleeding and represents one of the most challenging areas in hemophilia care. Most surgical and invasive procedures can be performed safely in patients with hemophilia with factor replacement therapy (intravenous infusion of the deficient factor at the appropriate dose during the adequate period of time). Despite the risk, the results of orthopedic surgery in patients with hemophilia are good; however, a favorable outcome depends on coordination between multidisciplinary team members. It is also crucial to ensure that orthopedic procedures in hemophilia patients are conducted in specialized centers by an experienced team [11].
The optimal level and duration of replacement therapy needed to prevent bleeding complications have not been established yet. Hermans et al., on behalf of the European Hemophilia Therapy Standardization Board, published a review article about replacement therapy for invasive procedures in patients with hemophilia [12]. They reviewed 35 clinical studies regarding replacement therapy for major invasive procedures. The preoperative target factor levels for hemophilia A and B were in the majority of studies (26/31) aimed at values >80 %. In the 27 studies that addressed postoperative trough levels for the first week, values were >70 % in eight studies, >50 % in 11, and >20–30 % in eight. For the second week, data were available in 18 studies, and levels were >50 % in four, >30 % in seven, and >10–20 % in seven.
Duration of treatment in 31 studies varied from 5 to 14 days in 19 of the studies, 15–21 days in six, and up to 28 days or longer in further six studies. Bleeding complications occurred in 131/1,328 or 10 % of major surgical procedures, most of them in the papers published before 1990.
As part of this comprehensive task, Hermans et al. also performed a survey in 26 European Hemophilia Comprehensive Care Centers, representing 15 different countries to develop consensus recommendations for replacement therapy [12]. All centers prior to major orthopedic surgery reported a target factor level of at least 80 %. In contrast to the literature, continuous infusion (CI) was used in procedures performed in nearly half of the centers. In most centers, factor levels were maintained above 80 % in the postoperative period. With bolus infusion (BI), two infusions per day were administered in order to maintain trough levels above 80 % during the immediate postoperative period (from day 1 to 5) and around 60 % in the late postoperative period (from day 6 to 14) – values that are higher than published targets. The duration of postoperative replacement therapy ranged from 12 to 14 days. When CI was used, the factor level was maintained at 80 % during the first five postoperative days and decreased to 30–40 % or 50–60 % between days 6 and 14. Before major orthopedic surgery, preoperative pharmacokinetic evaluation was performed in one-third of the centers and the recovery measured in more than half of them. Thromboprophylaxis with low-molecular-weight heparin was used in more than half, and antifibrinolytic treatment in more than two-thirds, of the centers [12].
Regarding synovectomy, Hermans et al. found four papers [12]. These four studies involved 158 patients undergoing 197 different procedures. Factor replacement always included a loading dose, ranging from 15 to 50 IU/kg in patients with hemophilia A and from 30 to 90 IU/kg in patients with hemophilia B, aiming at factor levels between 30–100 % and 30–90 %, respectively. Subsequent replacement therapy was either short (repeated bolus at full dose 8–12 h later and at half dose on day 2) or prolonged for 8 weeks using a prophylactic regimen (20 IU/kg three times per week – 2 weeks and 15 IU/kg two times per week – 6 weeks for hemophilia A and 30 IU/kg three times per week – 2 weeks and 25 IU/kg two times per week – 6 weeks for hemophilia B). No bleeding complications were reported. The survey from European Hemophilia Comprehensive Care Centers concluded that a target level of factor VIII (FVIII) between 80 % and 100 % was reported by 87.5 % of the centers. CI for this procedure was considered as an option by 62.5 % of the treaters. Treatment was continued for more than 7 days in a majority of the centers. Antifibrinolytics were used in 56 % of the centers. Our protocol for hemostatic cover in orthopedic surgery is shown in table 2.2.
Table 2.2
Factor level for invasive orthopedic procedures at our center
Procedure | Preoperative factor level (%) | Postoperative factor level |
|---|---|---|
Major orthopedic surgery | 80–100 | >80 % 1–7 days |
>50 % 8–15 days | ||
Surgical arthroscopic synovectomy | 80–100 | >50 % 1–7 days |
Radiosynovectomy | 80–100 | >50 % 1–5 days |
In conclusion, in major orthopedic surgery the preoperative factor levels should be 80–100 %, and in the postoperative period the minimal factor level should be above 50 % in the first week and 30 % in the late postoperative period. Nevertheless, the World Federation of Hemophilia (WFH) guidelines for perioperative plasma factor VIII levels recommend a preoperative level of 120 %, tapering down to 50 % at 14 days. In addition, a very interesting report from Wong et al. supports the fact that maintaining a high level of clotting factor replacement therapy (at least 80 % factor replacement over the first two postoperative weeks) throughout wound healing can result in lower infection rates, comparable to that of total knee arthroplasty (TKA) in patients without hemophilia [13].
2.2.2 Bolus Infusion (BI) and Continuous Infusion (CI) of Factor Concentrates
Most hemophilia centers use bolus infusion (BI) to replace coagulation factors for surgery [14]. Continuous infusion (CI) therapy was described initially in 1970 by McMillan et al. [15]. The idea was to maintain stable FVIII levels without the deep troughs that regularly accompany BI and expose the patient to the risk of bleeding (Fig. 2.1). However, it did not receive much attention because of technical issues related to the volumes required for infusion and concerns about the stability FVIII at room temperature and the potential for sepsis. Later it was observed that most FVIII concentrates are stable after reconstitution at room temperature for several days or even weeks [16, 17] and can be infused in small volumes of concentrated solution (with the addition of heparin to prevent local thrombophlebitis) via minipumps [18–20] to make CI a more accessible therapy.
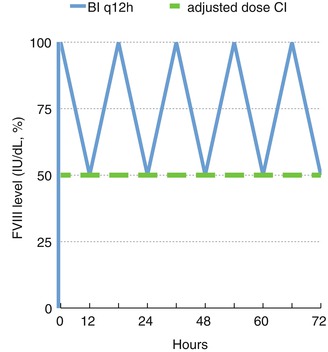

Fig. 2.1
FVIII levels in bolus infusion (BI) and adjusted continuous infusion (CI)
Martinowitz et al. described the most accepted method in hemophilia centers [18]. This method employs pharmacokinetic dosing and takes advantage of decreasing clearance of coagulation factor during CI. The simple protocol for this method is based on the following principles [18]:
1.
Pharmacokinetic evaluation prior to a planned CI is recommended. The most important pharmacokinetic parameter for calculating the ideal rate of continuous infusion is the clearance. In the absence of preoperative pharmacokinetic evaluation or, in particular, in emergency situations, the initial maintenance dose may be calculated using the mean of a hemophilia population-based clearance, which is approximately 3.5 mL/kg/h for FVIII and 4.5 mL/kg/h for FIX.
2.
The loading dose is calculated using in vivo recovery (IU/dL per IU/kg). A dose is selected that will raise the level to the desired minimum level appropriate for the surgical procedure requiring hemostatic replacement therapy, as mentioned before between 80 and 100 %.
3.
CI is initiated immediately following bolus administration of the loading dose. The initial rate is calculated using the clearance obtained in the preprocedure pharmacokinetic evaluation according to the following steady-state equation:
Rate of infusion (IU kg h) = clearance (mL kg h) × desired level (IU mL)
4.
From the second day, the CI maintenance dose is adjusted using the same equation according to actual clearance, which is calculated from the daily factor level measurements.
5.
Perioperative hemostatic demands may increase factor consumption beyond that expected. In order to prevent an undesired drop in the factor level, it is advisable to check factor activity 8–12 h after the start of CI and to increase the rate if necessary.
6.
In most patients who require treatment for more than 1 week, a significant decrease in FVIII clearance is observed during the first 5–6 days of CI, followed by a plateau at a significantly lower level than that observed in the first days postoperatively [14, 18, 21]. This allows one to reduce the maintenance dose progressively and results in a significant sparing of concentrate.
CI is a safe mode of treatment, more cost effective, and should be the treatment of choice for major surgery in hemophilia patients [18]. Batorova and Martinowitz reported a reduction in the bleeding rates and FVIII dosage (36 %) compared with the use of BI [14].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








