CHAPTER 24 Technique of Radiofrequency Denervation
INTRODUCTION
History
The use of electrical current to create lesions for the treatment of pain began in 1931, when Kirschner introduced it for the treatment of trigeminal neuralgia.1 In 1965, Mullan used direct current to perform percutaneous lateral cordotomy for unilateral malignant pain.2 Later that year, Rosomoff modified this technique and used radiofrequency (RF) current for this treatment.3 An RF current was found to produce more predictable, circumscribing lesions. Several years later, Sweet and Wepsic described their technique for the use of RF lesions of the Gasserian ganglion in the treatment of trigeminal neuralgia.4 In spinal pain, the use of RF current was reported in a series of cases by Shealy (1975).5 He described RF lesioning of the medial branch to treat lumbar facet joint pain. Subsequently, more options for the use of RF lesioning in various spinal pain syndromes were introduced into clinical practice.6–8 The introduction of a small-diameter (22-gauge) temperature monitoring electrode system by Sluijter and Mehta in 19819 increased the safety of the current method used.10 Since then, RF procedures have been widely adopted by many pain practitioners. In addition there have been many new technical developments, such as the improvement of the quality of C-arm image intensifiers. All these developments have contributed to a widespread use of RF lesioning.
Recently, a modification of the traditional RF method was introduced by Sluijter et al.11 They used the same output setting of the lesion generator that was used for making heat lesions as in RF lesioning, but they interrupted this output to allow generated heat to be washed out by thermal conductivity and circulation. This technique is called ‘pulsed radiofrequency’ (PRF) and is claimed to be nondestructive. Until now, there are no reports of any sensory or motor loss following this procedure. However, it is too soon to categorically recommend this technique because the mechanism of action is still unknown. Until several double-blind, randomized, controlled trials are conducted this new approach should not be routinely employed.
Physics
Radiofrequency current
An RF current is applied by an RF lesion generator through an electrode, which is insulated except for the most distal part. This exposed region is the active portion of the electrode. The RF current flows from the electrode tip to the dispersive ground plate, which is placed on the arm or leg of the patient and leads the current back to the RF lesion generator. RF current flows through tissue and results in an electric field. This electric field places an electric force on the ions within tissue electrolytes, causing them to oscillate at a high rate (i.e. 300 000 times per second).12 Tissue heating is created by frictional dissipation of the ionic current within the fluid medium, which heats the electrode.
Lesion size
The size of the lesion not only depends on the diameter of electrode and the length of the uninsulated electrode tip, but also depends on tip temperature. This was confirmed in animal studies by Cosman et al. in 1984.13 Bogduk et al. studied not only the size of the lesions, but also the shape of the lesion.14 They performed experimental lesions in egg white and fresh meat. They found that RF lesions do not extend distal to the tip of the electrode, but extend radially around the active electrode tip in a spheroidal shape. Based on these observations, Bogduk suggested that the best placement of the electrode tip was parallel to the target structure.
Other research methods involving computerized or mathematical modeling of similar experiments have been used. For example, Moringlane et al. studied experimental RF coagulation with a computer-based on-line monitoring of temperature and power.15 Vinas et al. also studied lesion size in vivo using fresh eggs and in vitro using the subcortical white matter of rabbits.16 Both investigators obtained results that were similar to those reported by Bogduk et al. Once equilibrium temperature is reached (after 20–40 seconds), the size of the lesion does not increase. However, some variables such as circulation effect and the tissue heat conductivity may also produce a variation in lesion size, but are unpredictable.13 Consequently, the lesion size may therefore be variable at different locations within the body.
Radiofrequency lesion generator
The modern RF lesion generating system has different functions, including a pulsed RF mode. There is continuous on-line impedance measurement to confirm continuity of the electrical circuit and to detect any short circuits. One of the key components of the system is nerve stimulator function. It is used to confirm the proper position of electrode tip (it indicates the electrode-to-nerve distance) and to permit minor adjustments. To ensure the proximity of the active tip to the sensory fibers, stimulation is performed at 50 Hz. The 2 Hz stimulation is carried out to detect extraspinal muscle contractions that occur when the needle is placed too close to a nerve root motor fiber. Voltage, current, and wattage during an RF procedure are also monitored. Finally, a generator monitors temperature using a thermocouple. This is an important lesion parameter, but the temperature is only measured at the tip of the electrode and not in the more peripheral zones of the lesion area. The usefulness of tip temperature to monitor the lesion size is minimized because the lesion size is dependent on local blood circulation. There is a rapid drop in temperature over the first few millimeters from the electrode tip.13,17
Effect of radiofrequency current on nerve tissue
The effect of RF lesions on nerve tissue is controversial. Letcher and Goldring, in 1968, investigated the effect of RF current and heat on the nerve action potential of the saphenous nerve in cats.18 Delta and C-fibers were blocked before the alpha-beta group by both RF current and heat. Uematsu,19 and subsequently Smith et al.,20 reported quite disparate results. Smith et al. created RF lesions of different temperatures by placing an electrode into the lumbar intervertebral foramina of dogs.20 Indiscriminate damage of both small and large fibers by RF current was observed. Uematsu conducted a histological analysis of feline sciatic nerves that sustained RF thermocoagulation.19 These studies have been criticized because large, 14-gauge electrodes were used; in addition, they were placed close to the dorsal root ganglion during open surgery. However, these methods are not comparable to the technique which is commonly used in clinical practice today, since 22-gauge needles where not available at that time.21 In response to this critique, de Louw et al., in 2001, tried to investigate morphological effects of RF lesions as they develop in normal clinical situations.21 A percutaneous RF lesion adjacent to the dorsal root ganglion (DRG) of goats was created with a 22-gauge electrode at a tip temperature of 67°C. Immunocytochemical changes indicative of proliferation and regeneration was observed 2 weeks after the RF lesion was created. These alterations occurred outside the DRG, without interfering with the function of large myelinated fibers.
Effect of pulsed radiofrequency current on nerve tissue
Recently, the importance of heat as the definitive mechanism of action RF lesioning has been questioned.11 In 1998, Sluijter et al. investigated isothermic RF lesioning also termed pulsed RF (PRF) treatment.11 Ostensibly, PRF generates its therapeutic effects independent of thermal factors. The pulses are given at a rate of 2 Hz and last 20 msec. The rest period is 480 msec, and this allows the generated heat to be washed out by thermal conductivity and circulation.
The mechanism by which pulsed RF treatment effects symptom relief remains unknown. In 2002, Higuchi et al. undertook a study to elucidate this mechanism. They attempted to identify spinal cord neuron activation following the exposure of the dorsal ganglion of rats to PRF currents.22 A significant increase in the number of cFos-immunoreactive neurons in the superficial laminae of the dorsal horn was observed. This finding indicates that PRF current activates pain-processing neurons in the dorsal horn and that this effect is not mediated by tissue heating.
This technique is considered to be a safer method of treatment because until now the observations showed no signs of neurodestruction and thus neurological side effects.11,23 Cahana et al., in 2003, compared the acute effects of pulsed versus continuous RF energy on impulse propagation and synaptic transmission in hippocampal slice cultures and on cell survival in cortical cultures of rats.24 In both systems, they found an induction of distance-dependent tissue destruction under the stimulating needle (namely, an inhibition of evoked synaptic activity), but this was more pronounced in the continuous RF group. Thus, they concluded that the acute effects of PRF are more reversible and less destructive in nature than the acute effects of classic continuous RF mode.
RADIOFREQUENCY DENERVATION IN THE CERVICAL REGION
Cervical percutaneous radiofrequency denervation of the facet joints
Relevant anatomy
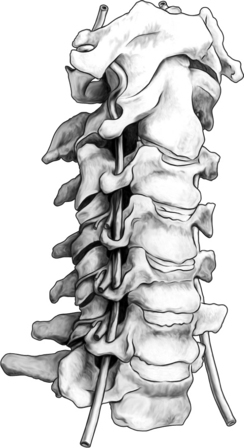
The cervical zygapophyseal joints are innervated in the same way as the lumbar zygapophyseal joints (Fig. 24.1). The cervical segmental nerves divide into the primary anterior ramus and the primary posterior ramus on exiting the neuroforamen. Immediately, the posterior branch divides into a lateral and a medial branch in the cervical intertransverse space. The medial branches of C4 to C8 run dorsally and medially through the ‘waist’ of their respective articular pillars. They innervate the facet joints at the level of the exiting segmental nerve and at the level below. Because of this multilevel innervation of the facet joints, usually the medial branch procedure is performed at several levels. Furthermore, the medial branches give deep branches to multifidus muscles and superficial branches to semispinal cervical muscles.
Procedure
Sedation is not employed because of the need for continuous communication between practitioner and patient. Several approaches to reach the medial branch of the dorsal ramus at the upper and middle cervical area can be used.
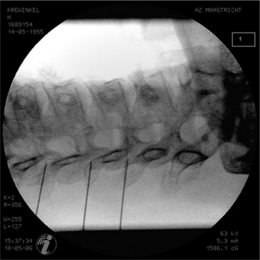
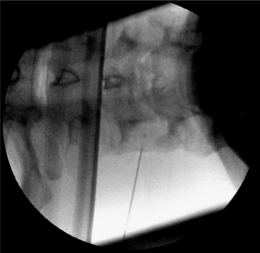
The most commonly used approach by these authors is the posterolateral approach.25 For this technique, the patient is positioned supine on the operating table, which is tolerated well. The occiput is placed on a headrest and the cervical spine is slightly extended. The C-arm is positioned in a moderately oblique (±30°) location to secure a safe distance between the electrode tip and the exiting segmental nerve. In this position the gantry angle is parallel to the axis of the intervertebral foramen that is upward and slightly caudal. In this position the segmental nerves exit in a plane approximately perpendicular to the monitor screen. The degree of obliquity should be such that the projection of the pedicles is seen a little anterior to 50% of the vertebral body (Fig. 24.2). In the frontal plane, there should be a small angle of the C-arm with the transverse plane. This provides clear visibility of the intervertebral discs and the neuroforamina. In this projection, the medial branch runs over the base of the superior articular process, which is easily viewed. Entry points are marked on the skin, somewhat posterior and caudal to the target points as seen on the monitor, i.e. dorsal from the posterior border of the facet column and slightly caudal. Care should be taken to ensure that the entry point is not too anterior in the neck to avoid important vascular structures such as the carotid artery. The first needle (preferably the most cranial) is inserted in a horizontal plane and in a slightly cranial direction not deeper than 2 cm, so that the needle point is in line with the target point. Then the needle is carefully advanced anteriorly and cranially until bone contact is made with the facet column at the target point (see Fig. 24.2).
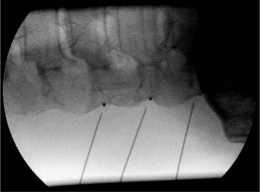
The position of the C-arm in the AP direction should confirm the position of the needle tip adjacent to the concavity (‘waist’) of the articular pillars of the cervical spine at the corresponding level (Fig. 24.3). Once the first needle is in the proper position, the other needles are introduced in the same way as described above. The authors prefer to take advantage of the first needle serving as an indicator for the direction and depth for subsequent needles, which accounts for their recommendation of placing all needles before applying RF rather than lesioning a single medial branch and then placing the next needle. The technique is identical for the facet joints from C3–4 until C6–7. The direction of the needle at the C2 level is different. It should be oriented toward the small branches that innervate the C2–3 facet joint and not toward the medial branch, which is the greater occipital nerve. For the RF treatment of this facet joint, the needle should be placed at the arch of C2 at the level of the upper border of foramen C3.
When optimal anatomical localization of the needles transpires, an electrical stimulation is performed to confirm correct needle position, i.e. parallel to the medial branch. This can be accomplished by identifying the stimulation thresholds. First, an electrical stimulation rate of 50 Hz should be given and should elicit a response (tingling sensation) in the neck at less than 0.5 volts. Next, a stimulation at 2 Hz is performed to confirm accurate needle position. Contractions of the paraspinal muscles will be noticed. Muscle contractions in the arm indicate needle placement too close to the exciting nerve root. In that case the needle should be repositioned more posteriorly. Once proper positioning of the needle is confirmed, the medial branch of the dorsal ramus is anesthetized with 1–2 mL local anesthetic solution (lidocaine 1–2%). An 80°C radiofrequency thermal lesion is made for 60 seconds at each level. To date, there have been no reported complications from cervical facet joint denervation when the procedure is methodically performed.26
Some postoperative burning pain is described in >30% of patients.27 It disappears spontaneously after 1–3 weeks. One should always bear in mind that there is risk of puncture of the vertebral artery when the needle is positioned anterior to the foramen in the posterolateral approach.
A second approach, which is more popular in the United States, is the posterior approach of the facet joint. This was first introduced by Lord et al. in 1995.28 In this technique, the patient is positioned prone on the operating table, with the head flexed (about 5–10°) and with the face resting on a padded ring. This is a ‘tunnel vision’ technique, in which the target points are the posterior aspects of the waists of the articular pillars at the levels to be blocked and are the same as the entry points. The needle is introduced from posterior of the neck to make contact with each of the two nerves supplying the painful joints. When bone contact is made, the C-arm is turned to give a lateral view in which the needle tip should be seen in the posterior aspect of the waist of the articular pillar. A deviation toward the midline should be avoided because of risk of penetration into the epidural and subarachnoid space. Subsequently, the needle is incrementally advanced until the tip is projecting at the center of the pedicle. Then, a slightly lateral deviation should be made so that the needle tip is in the most lateral aspect of the articular pillar in the posteroanterior view. When this position is confirmed, stimulation is performed as described above to determine the 50 Hz and 2 Hz thresholds. If these are acceptable, local anesthetic is injected and an RF lesion (80°C for 60 sec) is made. At each location two lesions are made: the second one after a rotation of the RF needle over 90° from its first position.
Equipment
Different radiofrequency lesioning probes can be used. A SMK-C5 (Sluijter-Mehta Kit, 22-gauge, 50 mm) cannula with a 4 mm active tip or, alternatively, an RCN-6 (24-gauge, 60 mm) needle is used. The advantage of an SMK needle is its ability to measure temperature, but there is the risk of displacement of the needle while inserting the RF probe. The RCN-6 electrode has a connecting tube that allows injection of local anesthetics before lesioning, minimizing potential displacement of the needle once it is positioned. There is no temperature measurement while using the RCN-6 electrode, but this is not thought to be a critical issue, although recently Buijs et al. reported that lesion size is more predictable while measuring the temperature.29 A 10 cm, 22-gauge SMK-electrode with a 4 mm tip is used for the posterior approach. With an SMK probe, an 80°C radiofrequency thermal lesion is made for 60 seconds at each level. With a RCN-6 needle, 20 volts over 60 seconds is applied to the electrode, which should heat the electrode tip to the correct temperature.
Postprocedure care
The patient is allowed to go home immediately after the procedure. A feeling of dizziness and vertigo is frequently described, especially after the higher median branch blocks. RF of the third occipital nerve secondarily can partially block the upper cervical proprioceptive afferents and can result in transient ataxia and unsteadiness.30,31 When this situation can possibly occur, patient should be advised not to drive a car or handle other dangerous machinery for the initial 24 hours following the procedure.
Cervical radiofrequency lesioning of the dorsal root ganglion
Procedure
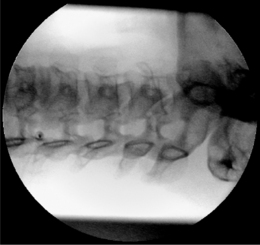
Again, this technique is performed without any sedation because of the need of cooperation of the patient. The radiofrequency lesioning of the dorsal root ganglion (RF-DRG) is only performed after a positive diagnostic segmental nerve block (usually several diagnostic blocks are performed). For this procedure, the patient is lying supine on the operating table with a slight extension of the neck. Firstly, the C-arm should be positioned obliquely so that the first three contralateral pedicles are projecting just posteriorly to the anterior line of the vertebral bodies. Secondly, the intervertebral discs should be clearly visible. To do so, the C-arm is orientated in the frontal plane. One should adapt C-arm positioning to have an optimal view of the intervertebral foramen of the level one wants to treat. The axis of the intervertebral foramen points 25–35° anteriorly and 10° caudally. The inclination of the C-arm in the frontal plane should be such that there is no double contour in the caudal aspect of the foramen. Then the target point is identified: it is posterior in the neuroforamen between the caudal and the middle third. This dorsal position is chosen in order to avoid possible damage to the motor fibers of the segmental nerve and to the vertebral artery, which runs anterior to the ventral part of the foramen. The entry point is marked on the skin and is the same as the target point. This technique is a ‘tunnel vision’ technique what means that the entry of the needle is in the direction of the X-rays.32 The needle is than projected on the screen like a dot and the field of vision can theoretically be narrowed down to a tunnel-like diameter (Fig. 24.4). After injection of local anesthetic (lidocaine 1%) into the skin with a 22-gauge subcutaneous needle, a 22-gauge spinal needle or, preferably, a needle with a connecting tube is inserted into the superficial layers of the skin, in the direction of the X-rays so that the electrode is projected on the screen as a dot. Subsequently, the needle is advanced carefully and every step is checked with fluoroscopy. There is the possibility of inadvertent puncture of the segmental nerve. That is why an early check in the anteroposterior (AP) view is done. In this view, the endpoint is the point where the tip of the needle is projecting 1–2 mm lateral to the lateral border of the facet column. Thereafter, only 0.2–0.5 mL of water-soluble dye (Iohexol 240 mg/mL; Omnipaque® 240) is injected to confirm the proper position of the needle tip close to the segmental nerve and to exclude an accidental intradural or intravascular position of the electrode. When this is confirmed, 0.5 mL of local anesthetic (lidocaine 1–2%) is injected. Alternatively, to perform a radiofrequency lesion, the cannula is advanced until the tip projects into the middle of the facet column (Fig. 24.5). Then the stylet is removed and 0.2–0.5 mL of dye is injected to confirm proximity to the targeted nerve root. The RF probe is now inserted through the cannula. After checking the impedance, electrical stimulation is started at a rate of 50 Hz. The patient should feel a tingling sensation. If the stimulation threshold is felt under 0.4 volts, the needle is withdrawn until the threshold is between >0.4 and <0.65 volts. At the C2 level, a suboccipital tingling has to be confirmed by the patient. Next, the frequency is changed to 2 Hz, and the patient is observed for muscle contractions in the arm and/or hand. These should not occur below a voltage of 1.5 times the 50 Hz threshold. When the thresholds are acceptable, 0.5–1.0 mL of local anesthetic (lidocaine 1–2%) is injected. Subsequently, an RF current is led through the electrode in order to increase the temperature at the tip slowly to 67°C for 60 seconds. As mentioned above, there is the tendency toward using pulsed RF current when treating the DRG because it is believed to be less destructive. With this technique, the stimulation at 50 Hz is started when the needle tip is projecting 2 mm medially to the lateral border of the facet column. An ideal threshold is obtained at <0.3 Hz. Then, a conventional 2 × 20 msec/sec, 45 volt and 120 sec. pulsed RF procedure is performed. The pulsed RF treatment can be repeated a second time if the 50 Hz threshold is lower than the initial threshold after a slight repositioning of the needle.
To perform a diagnostic block and (pulsed) RF procedure of the C2 DRG, the C-arm is positioned straight lateral because of the different anatomy at this level. There is no facet joint and there are no major blood vessels in the direct neighborhood that should be considered. The target point of the DRG of C2 is about 3 mm posterior to the tip of the dome-shaped space between the laminae of the C1 and C2 vertebrae (Fig. 24.6). After injection of a local anesthetic, the needle is inserted and advanced in a tunnel vision technique. The depth of the needle is checked in the AP position (Fig. 24.7) and the needle tip should be projecting halfway across and posterior to the C1–2 joint using a through-the-mouth view. The next steps are identical to those described above for the C3 to C7 DRG.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree