CHAPTER 108 Neuraxial Drug Administration to Treat Pain of Spinal Origin
INTRODUCTION
Advances in neurobiology serve as the basis for current and evolving implantable pain modalities, consisting of neurostimulation and neuraxial drug administration systems.
Both neurostimulation and intrathecal drug administration systems are reversible and nondestructive neuromodulatory techniques that can reduce pain of spinal origin. Neurostimulation is preferred over neuraxial drug delivery when the patient’s problem is amenable to both techniques because drug delivery systems require regular medication refills. Neuraxial medication delivery is indicated when side effects of systemic analgesics limit the ability to deliver effective doses of medications. Prager and Jacobs provide a comprehensive review of patient selection for these neuromodulatory techniques.1
RATIONALE
The discovery of opioid receptors2,3 provided a rational basis for the delivery of opioid drugs intraspinally. By 1979, reports of epidural4 and intrathecal5 opioid delivery in humans had entered the peer-reviewed literature.
Intraspinal infusions delivered drugs directly to opioid receptors, limited systemic exposure, and by decreasing the opioid dosage required for pain relief, generally reduced side effects, which facilitated the provision of greater analgesia. The benefits of short-term spinal analgesia, primarily for patients with intractable cancer pain, led to investigation of longer-term continuous subarachnoid opioid infusions for the management of both cancer pain6–17 and noncancer pain, such as that produced by failed back surgery syndrome.18–30 Pain specialists currently are successfully using opioids to treat patients with chronic noncancer pain, noting that such patients can benefit from sustained analgesia and better function without becoming addicted.31
The key to appropriate treatment of pain is proper diagnosis. Pain can be characterized as nociceptive (e.g., somatic pain), neuropathic (pain from nerve injury), or idiopathic. Pure nociceptive pain usually responds well to systemic opioids. Neuropathic pain responds to opioids at higher doses and often is responsive to a large number of antineuropathic medications (Table 108.1). Failed back surgery syndrome (FBSS) pain usually is a mixed type of pain that is both nociceptive and neuropathic.
Table 108.1 Updated pain treatment interventions and medications32,36
| Exercise |
| Meditation, relaxation, yoga, traditional biofeedback and neurobiofeedback |
| Over-the-counter medications (aspirin, traditional nonsteroidal antiinflammatory drugs) and cyclooxygenase inhibitors |
| Antineuropathic adjunctive medications (tricyclic antidepressants, selective serotonin reuptake inhibitors, GABAergic drugs, other antispasmodics, anticonvulsants, local anesthetics, calcium-channel blockers, substance P-depleting amines, α2 agonists, NMDA receptor antagonists, tramadol) and other adjunctive medications: corticosteroids |
| Physical rehabilitation: physical therapy, work hardening, occupational therapy, Pilates |
| Somatic and sympathetic nerve blocks |
| Cognitive and behavioral therapies |
| Opioid medications combined with adjuvants |
| Pure high-potency, time-released opioid medications with breakthrough short-acting opioid medications |
| Spinal cord stimulation if pain is segmental |
| Intraspinal infusion analgesia |
| Neurodestructive procedures |
In appropriately selected patients, intraspinal therapy has been refined through accumulated experience from treating tens of thousands of cases (more than 25 000 with implantable pumps32), improved drug delivery systems, and new pharmacologic approaches, making it an effective technique for the control of intractable pain.
INTRASPINAL DRUG DELIVERY SYSTEMS
Intraspinal drug delivery can be accomplished by a variety of means including percutaneous catheter, percutaneous catheter with subcutaneous tunneling, implanted catheter with subcutaneous injection site, totally implanted catheter with implanted reservoir and manual pump, and totally implanted catheter with implanted infusion pump.33 The choice of the system depends on the indication for intraspinal therapy, the need for bolus versus continuous infusion, the patient’s general medical condition, available support services, ambulatory status, life expectancy, and cost. In general, percutaneous tunneled catheters, external pumps, and implanted passive reservoirs can be more cost-effective when life expectancy is a matter of weeks to months. A fully implanted pump becomes economical if life expectancy is longer than 3 months.34
The first ‘permanent’ catheter for intraspinal drug delivery was developed by DuPen et al.35 in the 1980s. They adapted Broviac catheter technology to create an exteriorized, permanent, three-piece, silicone epidural catheter. The catheter was implanted in 55 cancer patients who had metastatic disease and intractable pain. After 3891 days of catheter use, there were no catheter infections and 18 minor side effects. The rate of hospitalization for pain control was decreased by 90% in these patients. In one series of 350 reported implantations of the DuPen catheter, there were 30 superficial catheter infections, 8 deep catheter infections, and 15 epidural or intrathecal catheter infections, representing a 15.1% infection rate. The DuPen catheter continues to be marketed for use with an external pump. It may represent a cost-effective alternative for patients with a short life expectancy, such as patients with severe metastatic disease to the spine. However, the DuPen catheter and similar external systems have limited applicability in treating noncancer pain of spinal origin.
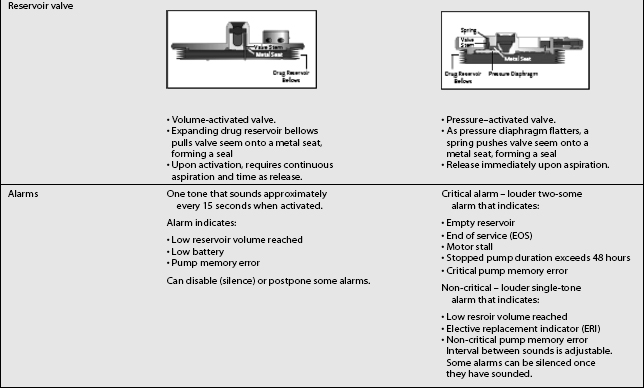
Two types of implantable drug delivery systems are marketed currently in the United States.34,36 The first commercially available implantable pump delivered medication at a fixed rate and consisted of two chambers separated by a flexible bellows in addition to a side port for bolus injections.3 Outflow was regulated by compressed Freon gas, so changes in altitude and temperature affected drug flow. Because the pump ran at a fixed rate, changes in the rate of medication delivery could be accomplished only by emptying the pump and refilling it with a different concentration of medication. This pump was approved by the Food and Drug Administration (FDA) for epidural administration of preservative-free morphine. The original pump has been superseded by a new model,2 which has a single raised septum and no side port. Although essentially the same, the new pump was modified by removing the side port to minimize the potential for overdose. A special needle with a needle shaft aperture and a closed needle tip is used to deliver fluid directly into the catheter rather than the drug reservoir.33 A second and third fixed-rate pump are now available.
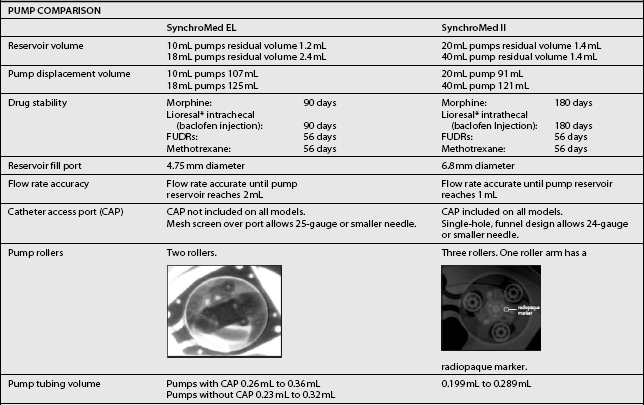
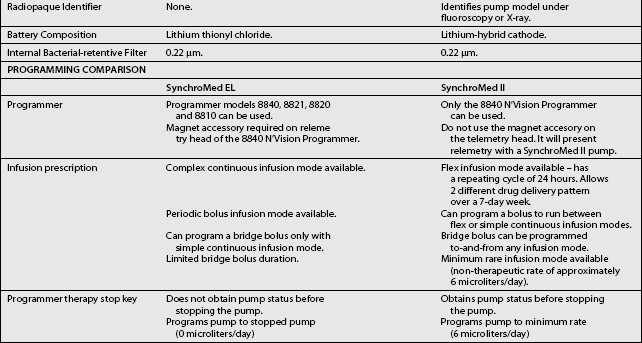
The third type of implantable delivery system is a programmable electronic pump powered by batteries, which last up to 7 years depending on flow rate.4 Two pumps are FDA approved and contain either 10 or 18 mL collapsible reservoirs, a volume activated valve and a pump or 20 or 40 mL reservoirs, a pressure activated valve and a pump, both of which push medication through a bacteriostatic filter and catheter. Comparison of these pumps is found in Table 108.2.
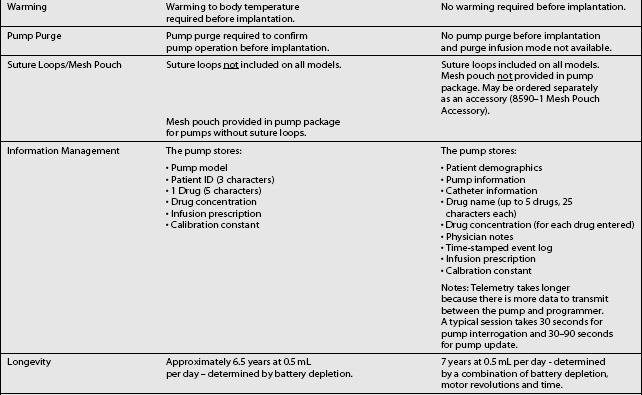
These pumps are FDA approved for epidural or intrathecal infusion of preservative-free morphine sulfate for chronic, intractable pain, and baclofen for chronic spasticity. Ziconotide was FDA approved for intrathecal use for pain in these pumps in 2004. Both pumps are programmed, using noninvasive telemetry to control medication concentration, volume, and dosage. The programmable feature allows flexible dosing options over time and permits precise dose titration. Both pump types require refilling under sterile conditions at least every several months, depending on flow rate.37
In deciding whether to implant a programmable pump or a fixed-rate pump, several factors should be considered, including their specific attributes. Table 108.3 compares the attributes of the two pump types. The programmable pumps provide greater flexibility of medication delivery and are more adjustable. However, a programmable pump is more expensive and needs to be replaced when the battery fails.
Table 108.3 Comparison of implanted pump characteristics39
| Consideration | Programmable pump | Fixed-rate pump |
|---|---|---|
| Cost | ||
| Environment factors | Will not run when cold‡ | Rate affected by pressure and temperature changes |
| Flexibility | Fixed-rate (some have a fixed rate selectable at time of implantation) | |
| MRI compatibility | Compatible§ | Compatible |
| Reservoir volume | 18 mL and 10 mL reservoirs or 20 and 40 mL reservoirs | |
| Other features |
* Hardware cost is one component of overall implantation costs. Other costs include operating suite, recovery room, radiology facility and professional fees, anesthesiology professional fees, surgical professional fees, and medications. When considering total cost, hardware cost represents only a small amount.
† Battery lifespan is estimated to be up to 7 years. Surgical costs are incurred for replacement.
‡ Only a factor in pump preparation, not at physiological temperatures.
§ Please see recently distributed guidelines.
PATIENT SELECTION AND SCREENING TRIALS
The literature is virtually unanimous in emphasizing the importance of appropriate patient selection if intraspinal pain therapy is to be successful.1 Patients with chronic pain are subject to neurophysiologic, emotional, and behavioral influences, which govern their perception of pain and of pain relief. Therefore, treatment of chronic noncancer pain is multidisciplinary, drawing on cognitive and behavioral psychological therapies, functional rehabilitation, orthopedic and neurologic surgery, medications, nerve blockade, neuroaugmentive and sometimes neurodestructive procedures. The pain treatment continuum in Table 108.1 lists these interventions and the antineuropathic medications currently used to treat intractable pain.32
Indications and contraindications for intraspinal opioid therapy appear in Table 108.4.34 Intraspinal drug delivery has been used primarily for patients with nociceptive pain, which has proved to be opioid responsive. Experience in intraspinal treatment of neuropathic pain is more limited, although several studies indicate that neuropathic pain may respond to intraspinal delivery of escalating doses of opioids, or to nonopioid medications.34,38 Finally, a screening trial allows both physician and patient to assess intraspinal drug delivery before committing to pump implantation.
Table 108.4 Updated indications and contraindications for intraspinal drug delivery34,36,39
| INDICATIONS | |
| Chronic pain with known pathophysiology | |
| Sensitivity of pain to medication being used | |
| Failure of more conservative therapy | |
| Favorable psychosocial evaluation | |
| Favorable response to screening trial | |
| CONTRAINDICATIONS | |
| Systemic infection | |
| Coagulopathy | |
| Allergy to medication being used | |
| Inappropriate drug habituation (untreated) | |
| Failure to obtain pain relief in a screening trial | |
| Unusual observed behavior during screening trial | |
| Poor personal hygiene | |
| Poor patient compliance | |
Numerous screening protocols exist. Trials can incorporate epidural or intrathecal administration, bolus injection, a series of injections, or continuous infusion, and they can be conducted on an inpatient or outpatient basis. Pure opioid or a mixture containing opioid can be administered. The duration of the trials varies from 24 hours to longer than 1 week. No protocol can be considered superior or definitive on the basis of current research. However, approximating the conditions of long-term therapy during the trial would seem to offer the best chance for assessing efficacy and tolerance. Table 108.5 compares the advantages of each trial technique.39
Table 108.5 Comparison of neuraxial trial techniques39
| Use of Current Systemic Medications during Trial | |
| Eliminates possible systemic abstinence syndrome (abstinence symptoms can confound results) | Can observe the effect and side effects of neuraxial medication without additive effect of systemic medications |
The choice of protocol is influenced by the patient’s overall condition, the physician’s preference and experience, the available facilities and resources, the practice environment, and the payer coverage. Medicare reimbursement, for example, requires ‘a preliminary trial of intraspinal opioid drug administration … with a temporary intrathecal/epidural catheter.’40 The question of epidural versus intrathecal administration continues to be debated, although no study has directly compared the two routes of administration. Although the epidural route is more convenient, an epidural dose must be roughly 10 times an intrathecal dose to provide equivalent analgesia.
Two questions are fundamental. Is the patient’s pain responsive to opioid therapy? Can the patient tolerate the planned drug and dosage?36 The physician and patient should agree in advance on the goals of the trial and on the measures to be used for assessment of the outcome. For example, if returning to work is a goal of long-term intraspinal drug therapy, the patient should be evaluated by a rehabilitation specialist during the screening trial. In general, candidates should not proceed to implantation unless their pain can be reduced by at least 50%.9,41 Behavioral observation during the trial adds to the information used for making decisions regarding permanent implantation.42
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree