There are a variety of cellular proteins and signaling pathways that are important in regulating cell reproduction or proliferation. A mutation that results in dysregulation of such pathways can increase cell proliferation, resulting in overgrowth of a cell type or an organ. Such pathways are frequently dysregulated in neoplasia. In some inherited conditions, when a single copy (one allele) of a gene that is important in regulating cell proliferation is mutated in the germ line, the result is an overgrowth phenotype, but when the second copy becomes mutated in a somatic manner (in a certain cell type), the result is the development of a tumor. Since these disorders are usually caused by one copy of the defective gene, they tend to be inherited in an autosomal dominant manner. The type of tissue or organ involved depends on the cell type in which the gene is expressed. In many syndromes, such as NF, the tissues of the musculoskeletal system are affected, resulting in obvious bone or soft-tissue abnormality. There is a risk of malignant progression, which develops over time as the cells are subjected to genetic damage (second hit), causing the loss of the normal copy of the causative gene. Recurrence of a deformity after surgery is not unusual, because the underlying genetic defect that causes abnormal cell growth cannot be corrected by any surgical procedure. Many children present with limb-length discrepancy, but most of these conditions will not be related to a syndrome and can be managed as described in
Chapter 28 on limb-length inequality. It is important to understand the various associated syndromes so that appropriate referrals can be made for nonorthopaedic problems.
Etiology.
NF is the most common single-gene disorder in humans, affecting 1 in 3000 newborns (
61,
62 and
63). NF1 is an autosomal dominant disorder with 100% penetrance, but onehalf of cases are sporadic mutations and are associated with an older-than-average paternal age. The most well-known patient who was presumed to have had NF, Joseph Merrick, also called the
Elephant Man, probably did not have this condition; his clinical profile better fits Proteus syndrome (
64). The
NF1 gene is located on chromosome 17 (
65). Its protein product,
neurofibromin, acts as a tumor suppressor (
66). There are also other potential genes located in introns within the
NF1 gene, whose functional significance is unclear.
Neurofibromin plays a role stimulating the conversion of Ras-GTP to Ras-GDP, and as such modulates activation of the Ras signaling system, which is involved in the control of cell growth (
67). Mutations in the
NF1 gene cause a disruption in its normal regulatory control of Ras signaling, giving affected cells an abnormal growth pattern. Neurofibromin is expressed at higher levels in the neural crest during development. Cells from the neural crest migrate to become pigmented cells of the skin, parts of the brain, spinal cord, peripheral nerves, and adrenals, thus explaining the common sites of abnormalities in the disorder. Disruption of the normal Ras signaling cascade is probably responsible for the malignant potential of this disorder. Only one of the two copies of the
NF1 gene is mutated in affected patients; however, tumors from such individuals have been found to have only the mutated gene because of loss of the normal copy (
68,
69,
70 and
71). The gene defect also gives a clue to potential novel therapies, because pharmacologic agents that block Ras signaling could be used to treat the disorder. Farnesyl transferase inhibitors block the downstream effects of Ras signaling activation and thus have the potential to be used in the treatment of some of the neoplastic manifestations of NF (
72,
73). Another therapeutic approach is the use of statin inhibitors, such as lovastatin, which is thought to regulate Ras signaling by the membrane binding of Ras (
52,
53).
Orthopaedic Manifestations.
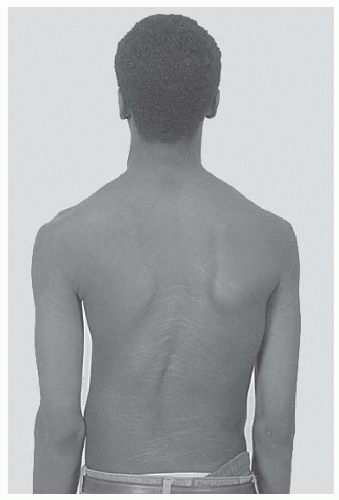
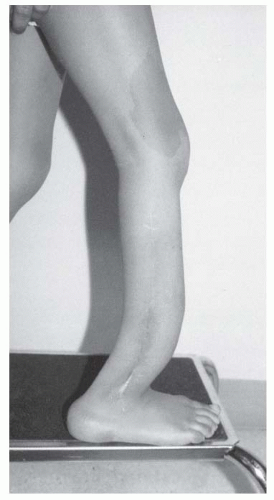
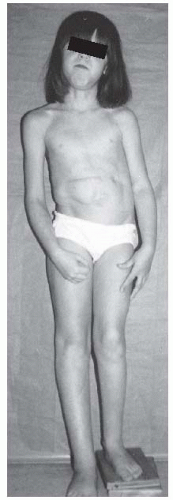
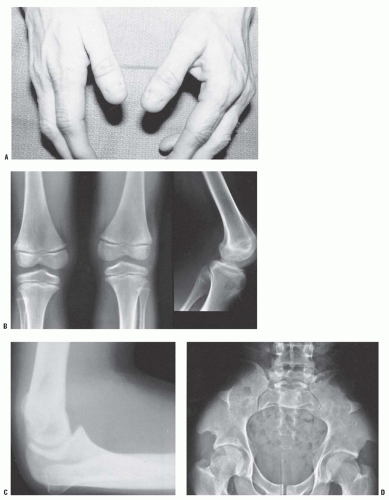
The orthopaedic manifestations of NF include scoliosis, overgrowth of the limbs, pseudarthrosis, and specific radiographic appearances of bone lesions. Patients with NF often exhibit overgrowth, ranging from a single digit to an entire limb and from mild anisomelia to massive gigantism. As such, the possibility of NF should be considered in a child with focal gigantism, such as macrodactyly. When NF is compared with the more symmetric idiopathic hemihypertrophy, there is disproportional overgrowth involving the skin and the subcutaneous tissue more than the bone (
Fig. 8-8)
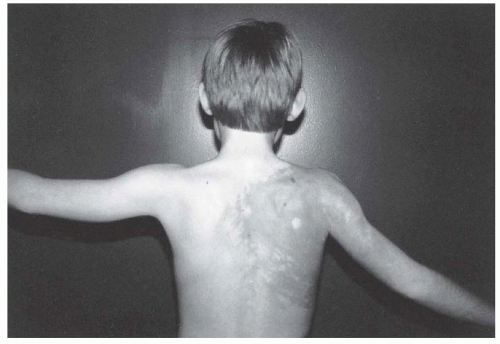
Scoliosis is common, and curves fall into two categories: a dystrophic curve and an idiopathic curve. Most curves in NF resemble idiopathic scoliosis curves and can be managed like any other idiopathic curve.
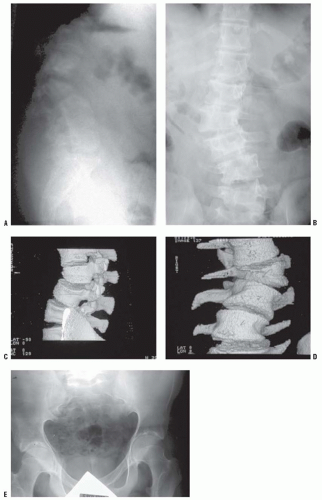
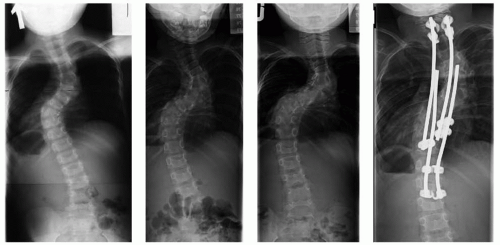
The dystrophic scoliotic curve is a short, sharp, single thoracic curve typically involving four to six segments (
Fig. 8-10) (
60,
74,
75,
76,
77,
78,
79,
80,
81). It is associated with deformity of the ribs and vertebrae. The onset is early in childhood, and it is relentlessly progressive. Curves that initially appear to be idiopathic in children under age 7 have almost a 70% chance of becoming dystrophic over time, although there may be subtle clues, for example, mild rib penciling (thinning of the ribs in a shape similar to a pencil point near the vertebrae), suggesting that the curve is actually dystrophic. The most important risk factors for progression are an early age of onset, a high Cobb angle, and an apical vertebra that is severely rotated, scalloped (concave loss of bone), and located in the middle-to-lower thoracic area (
78). The combination of curve progression and vertebral malformation mimics congenital scoliosis in appearance and behavior. Dystrophic curves are refractive to brace treatment. Sagittal plane deformities may occur, including an angular kyphosis (i.e., gibbus) and a scoliosis that has so much rotation that curve progression is more obvious on the lateral than on the anteroposterior radiograph (
78). In those with angular kyphosis, there is a risk of paraplegia. Dystrophic curves are difficult to stabilize, and it is best to intervene with early surgery involving both anterior and posterior fusion (
78,
82,
83 and
84). Kyphotic deformities are often the most difficult to manage surgically, and strut grafts across the kyphosis anteriorly may be necessary. In rare
severe cases, the spine can even seem to be “dislocated” because of the kyphosis and scoliosis. In cases with extremely severe deformity, halofemoral or halogravity traction may be necessary to safely straighten the spine to a more acceptable deformity without producing neurologic sequelae. Other reported techniques include inserting a bone graft without instrumentation and then gradually straightening the curve using a cast postoperatively (
85). In rare severe cases in which there is a vertebral “dislocation,” one can use instrumentation to achieve an overall alignment of the back, while leaving the vertebrae “dislocated” (
86). Unusual complications have been reported in the management of such dystrophic curves, such as a rib head migrating into the neural canal resulting in spinal cord compromise (
87).
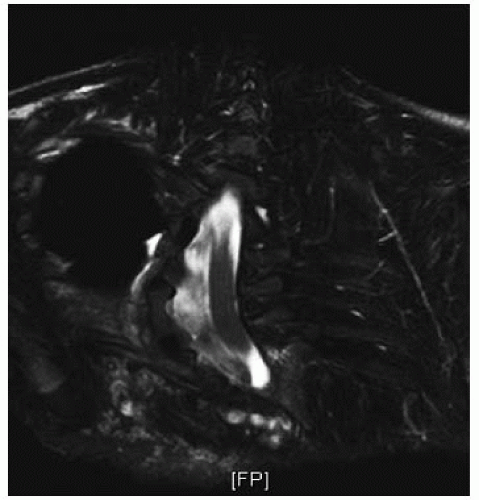
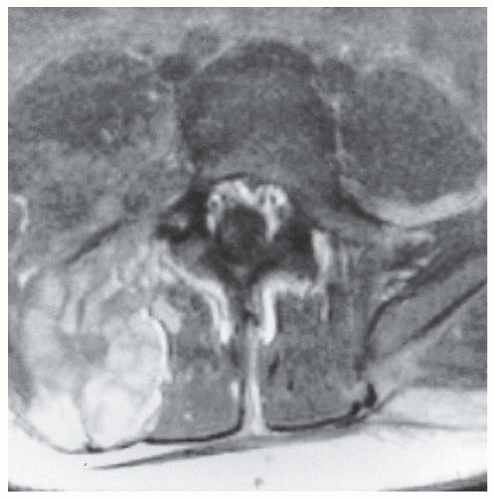
There can be several vertebral abnormalities evident on radiographs. These include scalloping of the posterior body, enlargement of the neural foramina, and defective pedicles, occasionally with a completely dislocated vertebral body (
88,
89,
90,
91 and
92). Such findings may mean that there is a dumbbell-shaped neurofibroma in the spinal canal, extending out through a neural foramina. The dura in NF patients behaves like the dura in patients with a connective tissue disorder, and dural ectasia is common, with pseudomeningoceles protruding through the neural foramina. Unlike neurofibroma, dural ectasia is an outpouching of the dura, without an underlying tumor or overgrowth of spinal elements (
Fig. 8-11) (
93,
94,
95 and
96). The incidence of anterolateral meningoceles was underestimated until asymptomatic patients were screened with MRI (
58,
97). The erosion of the pedicles may lead to spinal instability, especially in the cervical spine. In rare cases, this can even lead to dislocation of the spine (
98,
99). MRI and CT scans are helpful preoperatively in delineating the presence of defective vertebrae or dural abnormalities, and may assist in choosing the levels on which to place instrumentation.
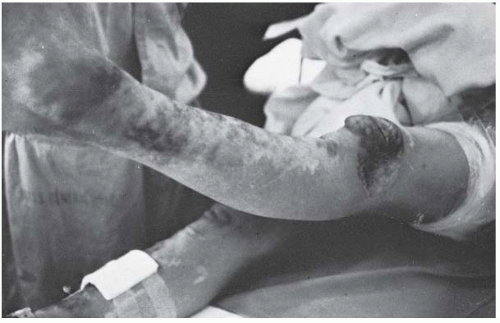
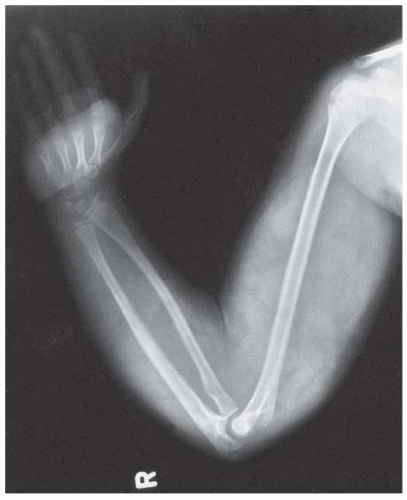
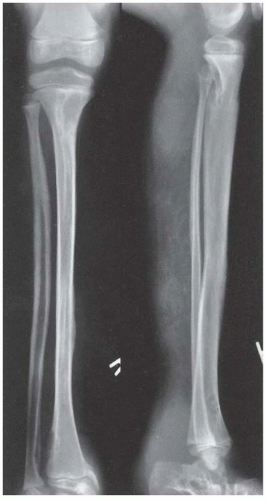
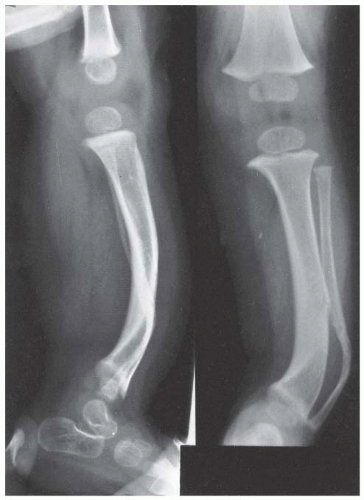
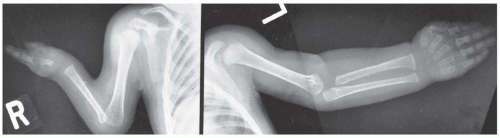
Pseudarthrosis of a long bone is typically associated with NF (
76). It usually affects the tibia, with a characteristic antero-lateral bow that is obvious in infancy (
Fig. 8-12) (
100,
101). Fracture usually follows, with spontaneous union being rare and surgical union presenting a challenge. An anterolateral bowed tibia is routinely managed with a total-contact orthosis to prevent fracture, although there are no well-designed studies showing that this is indeed effective. Intramedullary rod fixation seems to offer the best results for the initial management of a pseudarthrosis. Recent studies have shown the importance of achieving neutral tibial alignment in the healing of a tibial pseudarthrosis. The presence of an intact fibula is associated with a lower healing rate, perhaps because of associated tibial malalignment (
102). There is a hamartoma of undifferentiated mesenchymal cells at the pseudarthrosis site (
75), and in some cases, this is associated with loss of the normal allele of the NF1 gene (
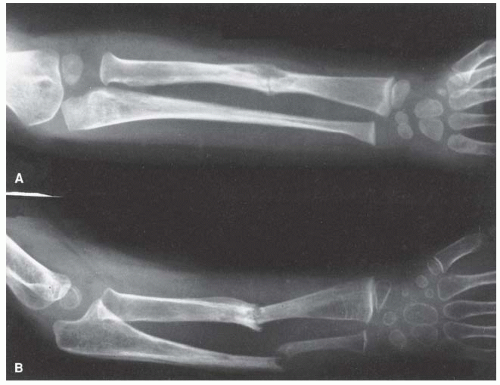
76). Neurofibromas have not been identified at the pseudarthrosis site. The pseudarthrosis process may affect the ulna, radius, femur, or clavicle (
77,
103,
104,
105,
106,
107,
108 and
109). In each of these locations, there is a course similar to that in the tibia, with bone loss and difficulty in achieving union (
Fig. 8-13). Not all pseudarthroses of the forearm require treatment (
110), but if they are symptomatic, the available options include proximal
and distal synostosis to produce a single-bone forearm, the use of a vascularized fibula graft, or resection of the pseudarthrosis with shortening of the forearm and internal fixation (
111). Pharmacologic approaches to the pseudarthrosis in NF are reported. A mouse model suggests the use of lovastatin, but the mouse does not develop pseudarthroses, only bowing of the bones, and as such human studies of this approach are needed (
53). Direct installation of BMP to the pseudarthrosis site may help in the achievement of union, but variable results are reported, and it is not known if the use of BMP in patients with an inherited premalignant condition has long-term harmful consequences (
80).
There are a variety of benign and malignant neoplastic lesions that affect individuals with NF1. Most neurofibromas do not require treatment, but symptomatic lesions may require excision. Plexiform neurofibromas that become symptomatic are very difficult to manage. Their vascularity and infiltrative nature make complete excision almost impossible, with a substantial risk of uncontrollable hemorrhage and neurologic deficit. Although speculative, the use of angiogenesis inhibitors, such as interferon, or experimental agents that modulate the effect of the causative gene mutation, such as farnesyl transferase inhibitors or statin inhibitors, may be beneficial (
88,
89).
The incidence of malignancy in NF is reported at rates ranging from under 1% to over 20% (
90,
91 and
92,
112,
113). The most common tumor location is in the central nervous system, with lesions such as optic nerve glioma, acoustic neuroma, and astrocytoma (
114). There is a risk of malignant degeneration of a neurofibroma to a neurofibrosarcoma. This process can occur in a central or peripheral neurofibroma (
115,
116,
117 and
118). It can be quite difficult to distinguish a malignant lesion from a benign one. CT scans show areas of low-enhancing density in neurofibrosarcomas (
119), but there are no studies confirming the sensitivity and specificity of this finding. Similar patterns can also be visualized using MRI. Routine surveillance for sarcomatous change is impossible because of the large number of neurofibromas. Lesions that increase in size or develop new characteristics should be investigated. There is a propensity for children with neurofibroma to develop other malignancies, such as Wilms tumors or rhabdomyosarcomas.
Hypertension as a result of renal artery stenosis or pheochromocytoma is reported regularly, as is a curious type of metabolic bone disease similar to hypophosphatemic osteomalacia (
120,
121). Hypertension is a major risk factor for early death (
113). Precocious puberty may occur because of an intracranial lesion (
103). Affected children are short, but tend to have large heads. Approximately 50% have an intellectual handicap. Although the mean IQ is low, the range of IQ is quite wide (
104). More than the low IQ, it is the difficulty in concentrating (which is common in this condition) that may interfere with the learning process (
105). Although it was hoped that lovastatin might help with concentration problems, a recent randomized trial suggests that this is not the case (
106).
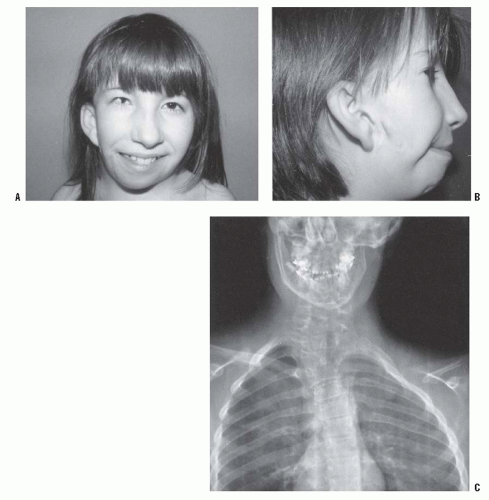
Beckwith-Wiedemann Syndrome. Beckwith
Wiedemann syndrome is a triad of organomegaly, omphalocele, and a large tongue (
107). The incidence is 1 in 14,000, and it is probably an autosomal dominant trait of variable expression. Patients are large, although this feature is not always noticed at birth (
108). The child is in the 97th percentile for size by 1 year of age. The tongue is gigantic at birth, and although it tends to regress, hemiglossectomy is sometimes needed. Omphalocele is common, and 15% of the babies born with omphaloceles have Beckwith-Wiedemann syndrome. The abdominal viscera are enlarged, and a single-cell hypertrophy
accounts for the large organs: in the adrenals, giant cortical cells; in the gonads, an increased number of interstitial cells; and in the pancreas, islet cell hyperplasia. This underlies the 10% risk of developing benign or malignant tumors. Wilms tumor is the most common.
Beckwith-Wiedemann syndrome is linked to chromosome 11p15, which is near the Wilms tumor gene (11p13) and the insulin-like growth factor gene (11p15.5) (
109). There may be some paternal genomic imprinting (
122,
123). The closeness of the Beckwith-Wiedemann gene locus and these embryonal tumor gene loci accounts for the dysregulation of the tumor-related genes and the associated overgrowth and higher incidence of tumors seen in this syndrome.
Pancreatic islet cell hyperplasia causes hypoglycemia. It is crucial that the neonatologist diagnose this syndrome early so as to prevent the consequences of hypoglycemia. If it is not managed properly, seizures occur at day 2 or 3. Central nervous system damage from the hypoglycemia leads to a cerebral palsy—like picture. The cerebral palsy—like findings confuse the diagnosis of this syndrome and make the management of these patients more complex. The diagnosis can occasionally be made prenatally by ultrasound (
124,
125).
The clinical feature that makes the orthopaedist suspect the presence of this disorder is the unusual combination of two otherwise common problems: spastic cerebral palsy and hemihypertrophy (
Fig. 8-14). The spasticity is thought to be a result of the neonatal hypoglycemic episodes, especially if accompanied by neonatal seizures, but spastic hemiplegia is most commonly seen. In general, children with cerebral palsy tend to be small; Beckwith-Wiedemann syndrome should be suspected if a large child has spastic cerebral palsy. Asymmetric growth affects about 20% of the patients. It is usually true hemihypertrophy, but it can be significant if the spastic hemiplegia affects the smaller side.
Children with Beckwith-Wiedemann syndrome are predisposed to a variety of neoplasms, most notably Wilms tumor. Abdominal ultrasounds at regular intervals until the age of 6, to screen for Wilms tumor, are advocated. A series comparing a screened population (ultrasounds every 4 months) with a population that was not screened showed that none of the children in the screened group presented with late-stage Wilms tumor, whereas one-half of the children who developed Wilms tumor in the nonscreened group presented with late-stage disease. This study suggests that screening every 4 months will identify early disease. However, a larger study is needed to determine whether screening improves patient survival (
125,
126). Other tumors types, such as alveolar rhabdomyosarcoma, can present in a new born (
100).
Scoliosis is common and usually behaves like an idiopathic spinal deformity, but there may be insignificant morphogenic variations, such as 13 ribs. It is managed in the same way as any idiopathic curve. Other orthopaedic findings include cavus feet, dislocated radial heads, and occasional cases of polydactyly (
127,
128). All of these can be managed the same as in sporadic deformities.
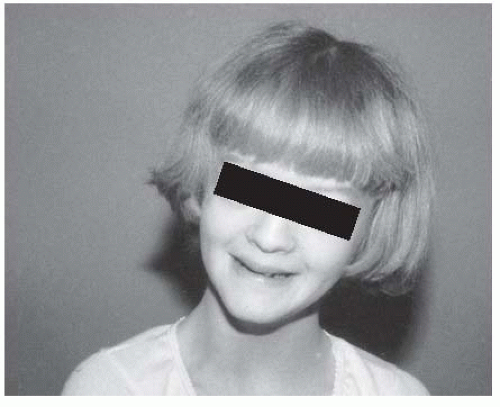
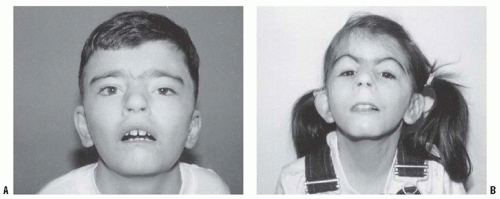
Russell-Silver Syndrome.
The patient with Russell-Silver syndrome is defined clinically as a short child with body asymmetry and a characteristic facial shape (129—131) (
Fig. 8-15). The diagnostic characteristics include (i) a birth weight ≤2 standard deviations below the mean, (ii) poor postnatal growth ≤2 standard deviations from the mean at diagnosis, (iii) preservation of occipitofrontal head circumference, (iv) classic facial features, and (v) asymmetric growth (
132). Poor feeding is also a common occurrence. The cause of the disorder is unclear; although some cases are associated with uniparental disomy, there is a suggestion of autosomal dominant inheritance, and there is some evidence implicating an abnormal intrauterine environment (
130,
131). The associated genitourinary malformations and the variation in the pattern of sexual maturation chemically (increased gonadotropin secretion) or clinically (precocious sexual development) suggest that hypothalamic or other endocrine disturbances may contribute to the pathogenesis. Affected children are small at birth and remain below the 3rd percentile throughout growth, with a marked delay in skeletal maturation. Body asymmetry with hemihypertrophy affects 80% of them. The asymmetry
averages approximately 2 cm at maturity, but can be as much as 6 cm. Regardless of the magnitude of the discrepancy, it is clinically more apparent because the child is small. The face is characteristically triangular and seemingly too small for the cranial vault. There have been several reports of variations in sexual maturation pattern and malformations of the genitourinary system.
Radiologic analysis discloses a remarkable array of orthopaedic findings, but it is not clear which form part of the syndrome and which are coincidental (133—137). Scoliosis is usually idiopathic. Hand and foot abnormalities include clinodactyly, polydactyly, and hallux varus. Developmental hip dysplasia, avascular necrosis of the femoral head, and slipped capital femoral epiphysis (SCFE) may be present. Many radiographic changes, such as the minor hand abnormalities, suggest a disturbed morphogenesis.
Treatment consists of managing leg-length equality. This can be difficult because individual growth curves may vary, the skeletal age is very retarded, and puberty may be very abnormal. It is easy to miss the appropriate timing for epiphysiodesis. Growth hormone has been administered in an attempt to improve stature. Although the use of growth hormone will increase growth velocity, it is not yet known whether the ultimate height is increased (
138).
Cytogenetic studies found anomalies on chromosomes 1, 7, and 17, but most patients have anomalies involving chromosome 7. However, no single causative gene has yet been identified. It is not known whether screening for Wilms tumor, as is performed in other forms of hemihypertrophy, is necessary. Despite early evidence that the insulinlike growth factor receptor, which plays a causative role in Wilms tumor, is involved in this syndrome, more comprehensive molecular genetic investigations have not found any abnormalities in this gene. However, there is a case report of Wilms tumor developing in an affected patient (
139), leading some to recommend screening for Wilms tumor in these patients as one would in any other hemihypertrophy.
Proteus Syndrome.
Proteus syndrome is an overgrowth condition in which there is a bizarre array of abnormalities that include hemihypertrophy, macrodactyly, and partial gigantism of the hands or feet, or both. The key to this diagnosis is worsening of existing symptoms and the appearance of new ones over time. There is a characteristic appearance to the plantar surface of the feet, often described as similar to the surface of the brain. Unlike in other overgrowth syndromes, an increased incidence of malignancy has not been reported in Proteus syndrome (
140,
141,
142,
143 and
144).
The cause of this syndrome is not known. Although there are case reports of familial occurrence, the vast majority of cases are sporadic (145—147). It is most likely due to a gene that is mutated in a mosaic manner (mutated in the affected tissues but not in the normal tissues), similar to McCune-Albright syndrome (polyostotic fibrous dysplasia). Such a mutation can occur very early in development in a single cell, which will divide to ultimately form various structures throughout the body.
The Proteus syndrome is named after the ancient Greek demigod who could change appearance and assume different shapes. The progressive nature of the deformities seen in this syndrome can lead to grotesque overgrowth, facial disfigurement, angular malformation, and severe scoliosis (
148). Joseph Merrick, called the
Elephant Man, is now believed to have had this syndrome rather than NF (
149).
The signs of Proteus syndrome overlap other hamartomatous overgrowth conditions, such as idiopathic hemihypertrophy, Klippel-Trenaunay syndrome, Maffucci syndrome, and NF. However, unlike these other syndromes, the features here are more grotesque and involve multiple tissue types and sites. Proteus can be differentiated from NF1 by the lack of caféau-lait spots and Lisch nodules (
150). A rating scale, which assigns points on the basis of clinical findings (macrodactyly, hemihypertrophy, thickening of the skin, lipomas, subcutaneous tumors, verrucae, epidermal nevus, and macrocephaly), may be used to assist in diagnosis (
151). However, the finding of worsening overgrowth features over time is usually sufficient to make this diagnosis.
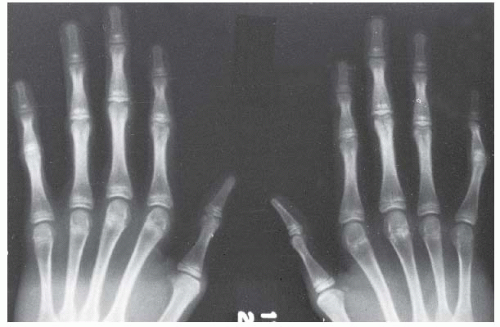
Most children who present with macrodactyly do not have it as part of Proteus syndrome. In these sporadic cases, an isolated digit is involved or, when multiple digits are involved, these are located adjacent to each other. Macrodactyly affecting nonadjacent toes or fingers or opposite extremities is almost always due to Proteus syndrome. There is a characteristic thickening and deep furrowing of the skin on the palms of the hands and soles of the feet. The array of cutaneous manifestations includes hemangiomas and pigmented nevi of various intensities, and subcutaneous lipomas (
Fig. 8-16). Varicosities are present, although true arteriovenous malformations are rare. There are cranial hyperostoses and occasionally exostosis of the hands and feet.
Macrodactyly seems to correspond to overgrowth along the terminal branches of a peripheral sensory nerve. Digital involvement in the hand favors the sensory distribution of the median nerve (
1). The index is the most frequently affected
finger, followed by the long finger and the thumb. It is the second toe that is most commonly macrodactylous. The regional sensory nerve is greatly increased in size, taking a tortuous route through the fatty tissue.
There is a wide range of orthopaedic deformities, including focal and regional gigantism, scoliosis, and kyphosis (
152,
153). Rather large vertebral bodies, known as
megaspondylodysplasia, are present (
154). Angular malformations of the lower extremities, especially genu valgum, are common. Because the genu valgum is often associated with restricted range of motion, flexion contractures, and pain in the joints, it is postulated that an intra-articular growth disturbance contributes to the angular malformation. Hip abnormalities that show up in roentgenographic tests, acetabular dysplasia for example, are frequently discovered in asymptomatic patients. Deformities in the hindfoot are frequent and are usually heel valgus, but congenital equinovarus and “Z-foot” deformities have also been described (
150,
153,
155).
Recurrences after various surgical intervention are very common. This is probably due to an underlying growth advantage in affected tissues that cannot be corrected operatively. Thus, musculoskeletal deformities caused by Proteus syndrome can be very difficult to manage. When the foot becomes difficult to fit into a shoe because of macrodactyly, it is best managed by ablation rather than debulking (
156). Anisomelia is best managed with epiphysiodesis. Osteotomies can correct angular malformations, but the decision to undertake surgical correction must take into account the possibility of a rapid recurrence of the deformity after corrective surgery (
152,
153). The use of growth modulation (e.g., 8-plate) to manage limb angular deformity is a rather promising approach (
120), but publications on the results of this approach are lacking. In some cases, a sudden overgrowth of the operative limb has been reported. There are anecdotal reports of soft-tissue procedures to “debulk” overgrown lesions; however, there are no series in the literature reporting results of these procedures, and our experience with them is that the results are only temporary. In rare cases, nerve or spinal cord impingement can occur. Nerve compression can be managed using decompression, but spinal cord compression is difficult, if not impossible, to successfully treat operatively (
157,
158). Scoliosis can occur and seems to be caused by overgrowth of one side of the spine (
159). Since mixed results are obtained from surgical treatment in this disorder, operative treatment should be reserved for individuals who have exhausted nonsurgical management. Sometimes, the operative procedures can be used as a temporizing measure, and patients may need to have repeat procedures performed throughout life.
Functional ability depends on the severity of the limb deformity and the presence of intracranial abnormalities (
143,
160). The life expectancy is unknown, but many adult patients have been reported. Intubations can be difficult because of overgrowth of structures surrounding the trachea.