Kyphosis is a curvature of the spine in the sagittal plane in which the convexity of the curve is directed posteriorly. Lordosis is a curvature of the spine in the sagittal plane in which the convexity of the curve is directed anteriorly. The thoracic spine and the sacrum normally are kyphotic, and the cervical spine and the lumbar spine normally are lordotic (
1). Although several authors have tried to define normal kyphosis of the thoracic spine and normal lordosis of the lumbar spine, these studies have shown much variability in what is considered normal (
2,
3,
4,
5,
6,
7 and
8). The ranges of normal kyphosis and lordosis change with increasing age and vary according to the gender and the area of the spine involved (
2,
3,
4 and
5). The degree of kyphosis or lordosis that is considered normal or abnormal depends on the location of the curvature and the age of the patient. For example, 30 degrees of kyphosis is normal in the thoracic spine but abnormal at the thoracolumbar junction.
The normal range of thoracic kyphosis is considered to be 19 to 45 degrees and that of lumbar lordosis, 30 to 60 degrees (
9). Measurements of kyphosis and lordosis are made from standard scoliosis radiographs with the patient standing with his or her knees locked, feet shoulder width apart, elbows bent, and knuckles in the supraclavicular fossa bilaterally. This will place the patient’s arms at approximately a 45-degree angle from the vertical axis of the body (
10). Thoracic kyphosis is measured on a lateral radiograph as the angle between the superior end plate of T2 and the inferior end plate of T12. Proximal thoracic kyphosis is measured from the superior end plate of T2 to the inferior end plate of T5. Middle and lower thoracic kyphosis is measured from the superior end plate of T5 to the inferior end plate of T12. The apex of normal thoracic kyphosis is the T6-T7 disc space (
11,
12). The thoracolumbar junction should have no kyphosis or lordosis (
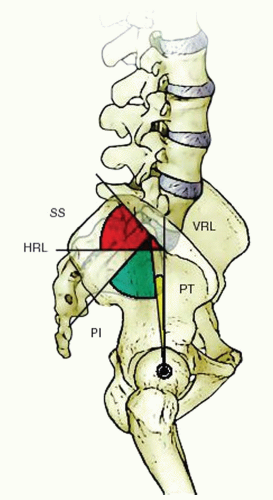
11). Lumbar lordosis begins at L1-L2 and increases gradually until the L3-L4 disc space. There is a reciprocal relationship between the orientation of the sacrum, sacral slope, and the pelvic incidence and the characteristics of lumbar lordosis and location of the apex of lumbar lordosis (
Fig. 19-1) (
13,
14,
15 and
16). A sacral slope of <35 degrees and a low pelvic incidence are associated with a relatively flat, short lumbar lordosis. A sacral slope of more than 45 degrees and a high pelvic incidence are associated with a long, curved lumbar lordosis (
14).
Initially, during fetal and intrauterine development, the entire spine is kyphotic. During the neonatal period, the thoracic, lumbar, and sacral portions of the spine remain in a kyphotic posture. Cervical lordosis begins to develop when a child starts holding his or her head up. When an upright posture is assumed, the primary and secondary curves begin to develop. The primary curves are thoracic and sacral kyphosis, and the secondary or compensatory curves in the sagittal plane are cervical and lumbar lordosis. These curves balance each other so that the head is centered over the pelvis (
2,
17,
18).
The ranges of normal thoracic kyphosis and lumbar lordosis are dynamic, progressing gradually with growth (
19). During the juvenile and adolescent growth periods, thoracic kyphosis and lumbar lordosis become more pronounced and take on a more adult appearance. Mac-Thiong et al. (
13) showed that pelvic incidence and tilt increased with growth but sacral slope remained stable.
Differences also exist between male and female spines (
6), and thoracic kyphosis and spine mobility are different in boys and girls: during the juvenile and adolescent periods (ages 8 to 16 years), girls have less thoracic kyphosis and thoracic spinal mobility than do boys of the same age (
3,
12). Thoracic kyphosis also tends to progress with age: from 30 to 70 years of age, women have a progressive increase in kyphosis, from a mean of 25 degrees to a mean of 40 degrees (
19). Men also show a definite progression with age, but at a lower rate.
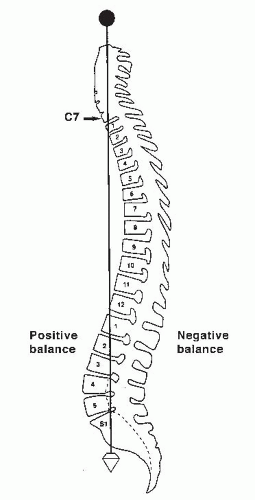
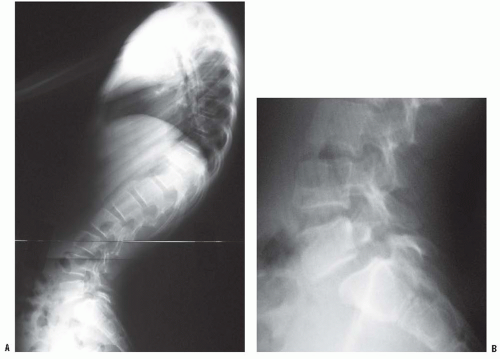
Normal sagittal balance is defined as a plumb line dropping from C7 and intersecting the posterosuperior corner of the S1 vertebral body (
Fig. 19-2). Positive sagittal balance occurs when the plumb line falls in front of the sacrum, and negative sagittal balance occurs when the plumb line falls behind the sacrum (
20).
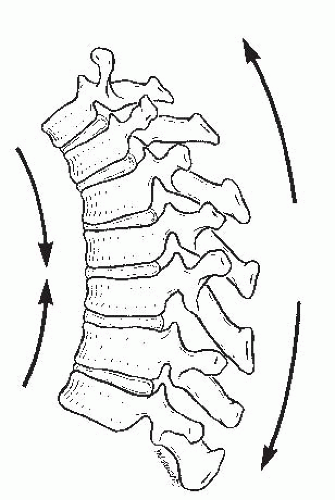
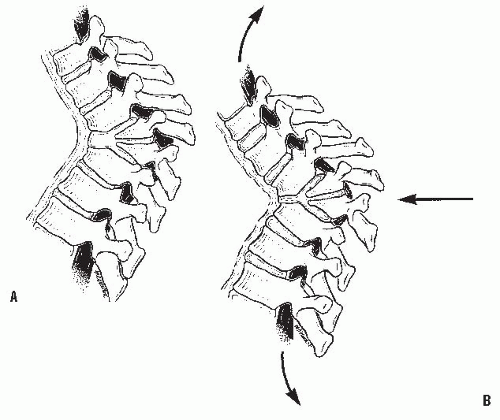
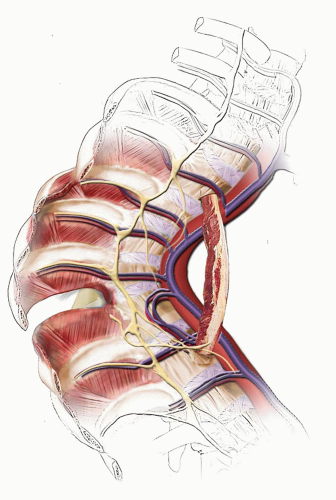
Different forces are exerted on the spine, depending on the presence of kyphosis or lordosis. In the upright position, the spine is subjected to the forces of gravity, and several structures maintain its stability: the disc complex (nucleus pulposus and annulus), the ligaments (anterior longitudinal ligament, posterior longitudinal ligament, ligamentum flavum, apophyseal joint ligaments, and interspinous ligament), and the muscles (the long spinal muscles, the short intrinsic spinal muscles, and the abdominal muscles). Alteration in function resulting from paralysis, surgery, tumor, infection, or alteration in growth potentials can cause a progressive kyphotic deformity in a child (
21). Both compressive and tensile forces are produced by the action of gravity on an upright spine (
Fig. 19-3). In normal thoracic kyphosis, the compressive forces borne by the anterior elements are balanced by the tensile forces borne by the posterior elements. In a lordotic spine, the compressive forces are posterior and the tensile forces are anterior. These forces of compression and tension on the spinal physes can cause changes in normal growth, and a growth deformity can be added to a biomechanical deformity to cause a pathologic kyphosis (
21,
22).
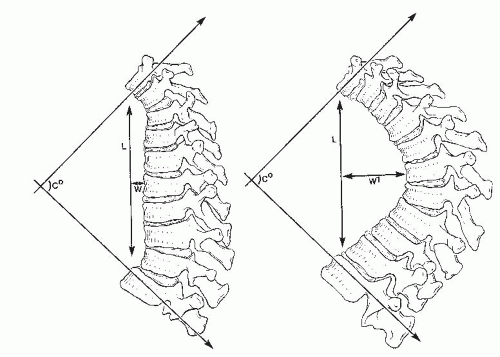
Voutsinas and MacEwen (
23) suggested that relative differences in forces applied to the spine are reflected more accurately by the length and width of a kyphotic curve than by just the degree of the curve. For example, curves that are longer and wider (farther from the center of gravity) are more likely to cause deformity in an immature spine (
Fig. 19-4). Winter
and Hall (
24) classified disorders that result in kyphosis of the spine. Only the more common causes are presented in this chapter; the other causes are discussed elsewhere in this book (
Table 19-1).
CONGENITAL KYPHOSIS
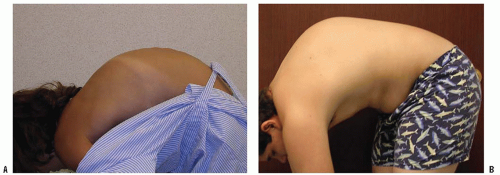
Congenital kyphosis is an uncommon deformity, but, despite its rare occurrence, neurologic deficits resulting from this deformity are frequent.
Congenital kyphosis occurs because of abnormal development of the vertebrae, including a failure of developing segments of the spine to form or to separate properly (
27). The spine may be either stable or unstable, or it may become unstable with growth (
28). Spinal deformity in congenital kyphosis usually progresses with growth, and the amount of progression is directly proportional to the number of vertebrae involved, the type of involvement, and the amount of remaining normal growth in the affected vertebrae (
28,
29).
Van Schrick in 1932 (
30) and Lombard and LeGenissel in 1938 (
31) initially described two basic types of congenital kyphosis: a failure of formation of part or all of the vertebral body and a failure of segmentation of part or all of the vertebral body. Winter et al. (
27,
32) developed the most useful classification of congenital kyphosis, which divides the deformity into three types (
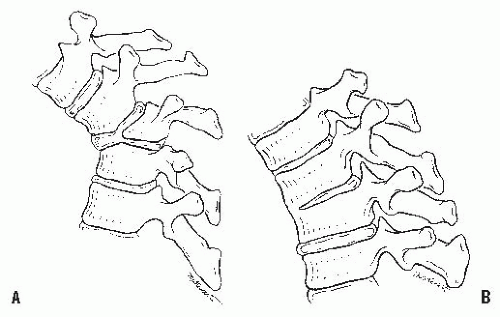
Table 19-2). Type I is failure of formation of all or part of the vertebral body (
Fig. 19-6A); type II is failure of segmentation of one or multiple vertebral levels (
Fig. 19-6B); and type III is a mixed form, with elements of both failure of formation and failure of segmentation.
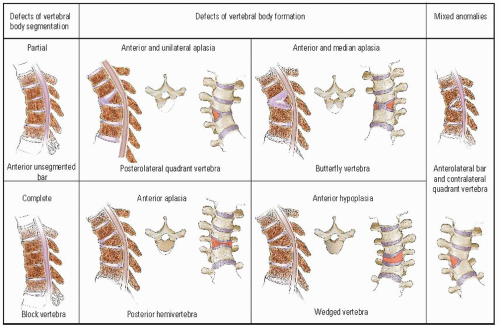
McMaster and Singh (
33) further subdivided this classification into types of vertebral-body deformity. Defects of vertebral-body segmentation consist of a partial (anterior unsegmented bar) or a complete (block vertebrae) failure of segmentation. Defects of vertebral-body formation are divided into four types: (a) posterolateral quadrant vertebrae, (b) butterfly
vertebrae, (c) posterior hemivertebrae, and (d) wedged vertebrae (
Fig. 19-7). Dubousset (
34) and Zeller et al. (
35) added a rotary dislocation of the spine, and Shapiro and Herring (
36) further divided type III displacement into types A (sagittal plane only) and B (rotary, transverse, and sagittal planes). Any classification can be subdivided further into deformities with or without neurologic compromise; this is useful for making treatment decisions because each type of congenital kyphosis has a distinct natural history and risk of progression.
Most of the vertebral malformations that cause spinal deformity occur between the 19th and the 30th days of fetal development (
28,
32,
37). The somatic mesoderm, which is devoted to the formation of the vertebral column and the rib cage, undergoes segmentation into 38 to
44 pairs of discrete, bilateral somites. The formation of a vertebra depends on contributions of cells from two separate and successive pairs of sclerotomes. This condensation of the paired sclerotomes occurs at approximately 5 weeks of gestation. If one side of the pair of sclerotomes fails to develop, a hemivertebra is formed, resulting in congenital scoliosis (
38,
39).
Tsou (
40) concluded that congenital kyphosis and congenital scoliosis occur during different periods of spinal development. He divided the development of the spine into an embryonic period (the first 56 days) and a fetal period (from day 57 to birth). During the embryonic period, failure of segmentation and aplasia of part of the vertebrae, resulting in hemivertebra formation, cause scoliosis, while congenital kyphosis occurs in the fetal period, during the cartilaginous phase of development (
40). Failure of formation occurs in this phase when the cartilaginous centrum of the vertebral body forms a functionally inadequate growth cartilage.
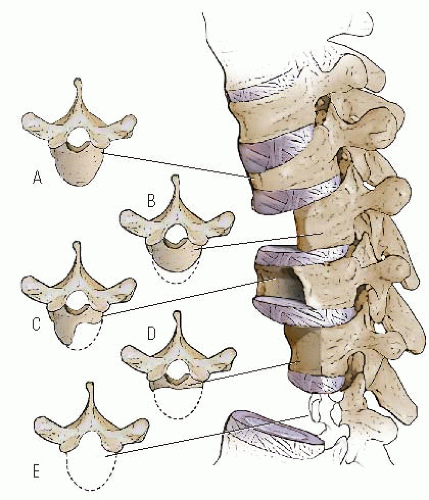
Failure of formation varies from complete aplasia (which involves the pars and the facet joints and makes the spine unstable) to involvement of only the anterior one-third to one-half of the vertebral body. This abnormal development is
thought to be the result of inadequate vascularization of the vertebral body during the fetal period, leading to hypoplasia or aplasia of the anterior vertebral body. If one side of the vertebra is involved more than the other side, scoliosis also may occur (
Fig. 19-8). Unlike hemivertebral anomalies that occur in the embryonic period because of maldevelopment of corresponding pairs of somites causing congenital scoliosis, posterior arch anomalies usually are absent in pure congenital kyphosis.
Failure of segmentation has been described as an osseous metaplasia of the annulus fibrosus (
40,
41) that acts as a tether against normal growth and causing spinal deformity. The height of the vertebral bodies is relatively normal, but the depth of the ossification of the annulus fibrosus varies. Ossification may be delayed, with a period of normal growth followed by spontaneous ossification. Kyphosis caused by a “segmentation defect” is believed to represent a developmental defect of the perivertebral structures (the annulus fibrosis, the ring apophysis, and the anterior longitudinal ligament) rather than a true intervertebral bar (
42).
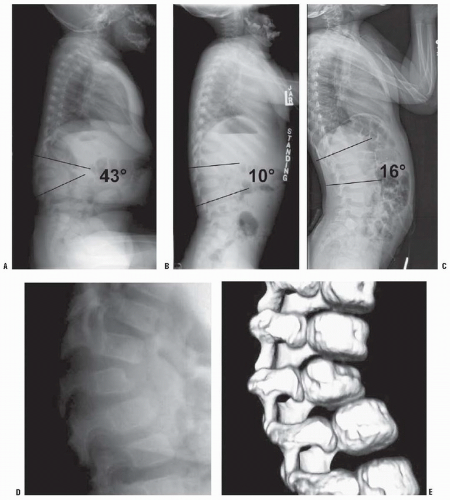
The natural history of congenital kyphosis is well known and based on the type of kyphosis: failure of formation (type I), failure of segmentation (type II), or mixed anomalies (type III). Congenital kyphosis tends to be progressive, with the greatest rate of progression occurring during the time of most rapid growth of the spine (birth to 3 years of age) and during the adolescent growth spurt. Winter et al. (
32) found that failure of formation (type I deformity) produces a much more severe kyphosis, with a rate of progression that averages 7 degrees per year, whereas type II deformities progress an average of 5 degrees per year. McMaster and Singh found the most rapid progression in type III kyphosis, followed by type I, because of involvement of posterolateral quadrant vertebrae. In their study, a type III kyphosis progressed at a rate of 5 degrees per year before 10 years of age and 8 degrees per year thereafter until the end of growth. Type I (failure of formation) kyphosis progressed 2.5 degrees per year before 10 years of age and 5 degrees per year thereafter (
33). Type I and III deformities are associated with a much higher incidence of neurologic involvement and paraplegia than are type II deformities. Neurologic problems occur more frequently in patients with type I and III deformities because they tend to have an acute angular kyphosis over a short segment, which places the spinal cord at higher risk for compression at the level of acute angulation. Type II deformities (failure of segmentation) rarely result in neurologic problems because involvement of several segments produces a more gradual kyphosis, and vertebral-body height usually is maintained with little or no vertebral-body wedging. The most frequent location of congenital kyphosis is T10-L1 (
32).
Patients with congenital kyphosis may have other anomalies. Intraspinal abnormalities have been reported to occur in 5% to 37% of patients with congenital kyphosis and congenital scoliosis (
43,
44,
45 and
46). A study by Bradford et al. (
47) indicated that this incidence may be even greater. They found that six of eight patients with congenital kyphosis had spinal cord abnormalities visible on magnetic resonance imaging (MRI). Although the proposed time of development of the deformity may be different from that of congenital scoliosis, other nonskeletal anomalies such as cardiac, pulmonary, renal, and auditory disorders or Klippel-Feil syndrome (
48,
49) can be associated with congenital kyphosis. McMaster et al. (
50) found an adverse effect on lung development and function caused by an increasing constriction of the rib cage and impairment of diaphragmatic movement. The more cranial the level of the congenital kyphosis, especially above T10, the more significant the effect on respiratory impairment.
Patient Presentation.
The diagnosis of a congenital spine problem usually is made by a pediatrician before the patient is seen by an orthopaedist. The deformity may be detected before birth on prenatal ultrasonography (
51) or noted as a clinical deformity in a newborn. If the deformity is mild, congenital kyphosis can be overlooked until a rapid growth spurt makes the condition more obvious. Some mild deformities are found by chance on radiographs that are obtained for other reasons. Clinical deformities seen in a newborn tend to have a worse prognosis than those discovered as incidental findings on plain radiographs. Physical examination usually reveals a kyphotic deformity at the thoracolumbar junction or in the lower thoracic spine. An attempt should be made to determine the rigidity of the deformity
by flexion and extension of the spine. A detailed neurologic examination should be done, looking for any subtle signs of neurologic compromise. Associated musculoskeletal and nonmusculoskeletal anomalies should be sought on physical examination.
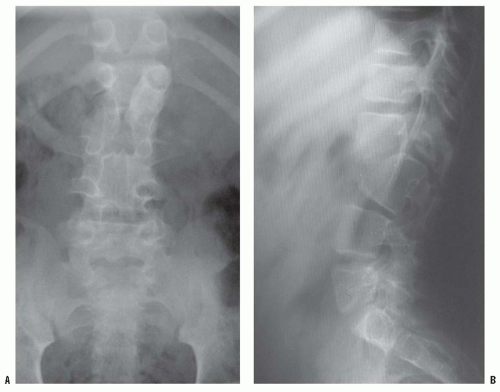
High-quality, detailed anteroposterior and lateral radiographs provide most information in the evaluation of congenital kyphosis (
Fig. 19-9). Failure of segmentation and the true extent of failure of formation may be difficult to detect on early films because of incomplete ossification. Flexion and extension lateral radiographs are helpful in determining the rigidity of the kyphosis and possible instability of the spine. Computerized tomography (CT) with three-dimensional reconstructions can identify the amount of vertebral-body involvement and can determine whether more kyphosis or scoliosis might be expected (
Fig. 19-10). CT scans can identify only the nature of the bony deformity and the size of the cartilage anlage. They do not show the amount of growth potential in the cartilage anlage, and therefore only an estimate of possible progression can be made. MRI should be obtained in most cases because of the significant incidence of intraspinal abnormalities. In addition, the location of the spinal cord and any areas of spinal cord compression caused by the kyphosis can be seen on MRI. The cartilage anlage will be well defined by MRI in patients with failure of formation (
Fig. 19-11); however, as with CT scans and plain radiographs, MRI cannot reveal how much growth potential is present in the cartilage anlage and can only help estimate the probability of a progressive deformity.
Congenital kyphosis, as well as associated renal problems, can be seen on routine prenatal ultrasonography as early as 19 weeks of gestation (
51). Myelograms have been used for documenting spinal cord compression but have been mostly replaced by MRI. If myelography is used, images should be taken with the patient prone and supine. Myelograms obtained in only the prone position may miss information about spinal cord compression because of pooling of dye around the apex of the deformity. Myelography can be used in conjunction with CT scanning to add to the diagnostic information obtained.
PROGRESSIVE ANTERIOR VERTEBRAL FUSION
Progressive anterior vertebral fusion (PAVF) is rare and is an uncommon cause of kyphosis in pediatric patients; however, if discovered late it may be confused with type II congenital kyphosis. Knutsson (
61), in 1949, was the first to describe PAVF in the English-language literature, and fewer than 100 cases have since been reported (
62,
63,
64,
65,
66,
67 and
68). Because the largest reported series (26 patients) was from the University Hospital of Copenhagen (
68), some have named this the Copenhagen syndrome. This condition is distinguishable from type II congenital kyphosis because the disc spaces and vertebral bodies are normal at birth and later become affected with an anterior fusion. Although the etiology is unknown, PAVF is probably a distinct clinical condition; however, it may represent a delayed type II congenital kyphosis.
Dubousset (
34) suggested that certain forms of type II congenital kyphosis (failure of segmentation) may be inherited. The patients have a failure of segmentation, with delayed fusion of the anterior vertebral elements, which is not visible on radiographs until 8 or 10 years of age. He described one family in which three individuals had delayed ossification and congenital kyphosis, and another family in which the grandmother, mother, and two sisters had the deformity. Kharrat and Dubousset (
62) also found this condition to be familial in 6 of 15 patients, and Van Buskirk et al. (
63) reported associated anomalies in 7 of 15 patients, including heart defects, tibial agenesis, foot deformities, Klippel-Feil syndrome, Ito syndrome, pulmonary artery stenosis, and hemisacralization of L5.
Neurologic deficits are usually not seen in patients with PAVF, but Smith (
63) reported one case of spinal cord compression resulting from an acutely angled kyphosis. Van Buskirk et al. (
63) and Dubousset (
28,
34) described five stages of PAVF: stage 1 is disc space narrowing, which occurs to a greater extent anteriorly than posteriorly; stage 2 is increased sclerosis of the vertebral end plates of the anterior and middle columns; stage 3 is fragmentation of the anterior vertebral end plates; stage 4 is fusion of the anterior and sometimes the middle columns; and stage 5 is development of a kyphotic deformity. Hughes and Saifuddin (
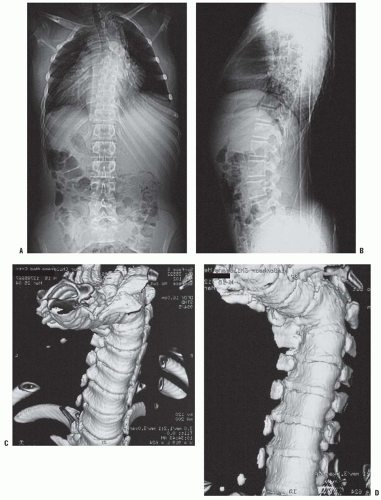
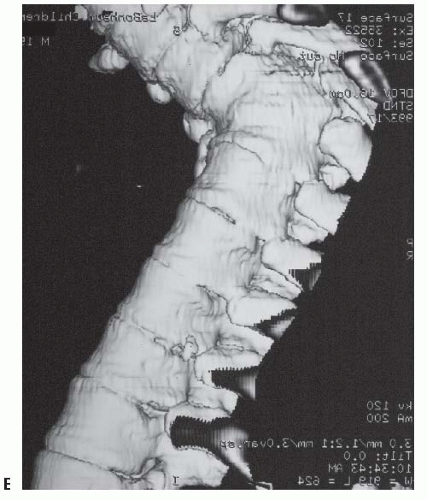
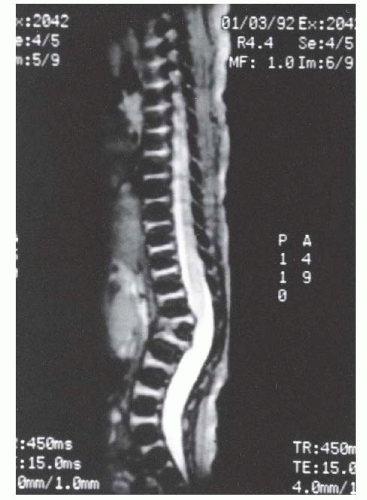
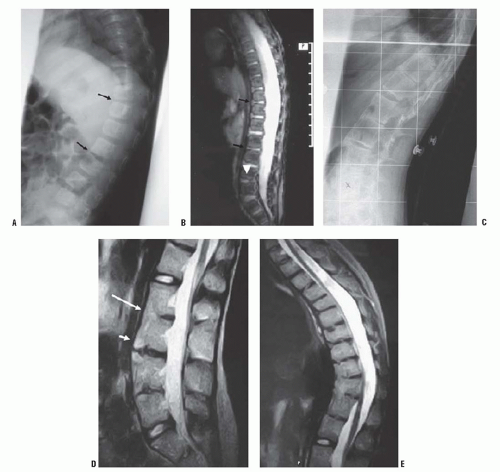
67) described the MRI appearance of PAVF in three patients: early anterior disc narrowing (
Fig. 19-18A), significant end-plate edema and fatty marrow changes (
Fig. 19-18B), and finally multilevel anterior fusion and disc obliteration (
Fig. 19-18C-E)
Kyphosis is the last stage in PAVF and is caused by the anterior disc space fusing while part of the posterior disc space remains open, allowing for continued growth in the posterior disc space and the posterior column. Bollini et al. (
65) found that patients with thoracic PAVF had a relatively good prognosis, whereas those with lumbar involvement had a poor prognosis. Involvement of the thoracic spine is better tolerated by patients than is involvement of the lumbar area because of the normal kyphotic posture of the thoracic spine. Therefore, nonoperative treatment is recommended for most thoracic PAVF deformities. For PAVF in the lumbar spine, a posterior spinal fusion is indicated in stages 1, 2, and 3. In stages 4 and 5, the kyphotic deformity has already occurred in a normally lordotic lumbar spine. Posterior fusion will only stop progression of kyphotic deformity. If normal sagittal alignment is to be obtained, an anterior osteotomy followed by posterior fusion and instrumentation is recommended (
61,
62,
63,
64,
65,
66,
67 and
68).
SEGMENTAL SPINAL DYSGENESIS
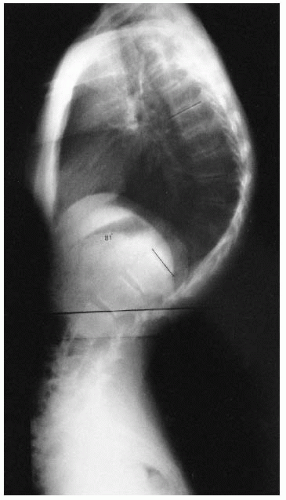
Segmental spinal dysgenesis is a congenital anomaly of the lumbar or thoracolumbar spine, consisting of focal agenesis or dysgenesis of the spine, and resulting in severe spinal stenosis and instability (
70). A progressive kyphosis occurs at the site of segmental spinal dysgenesis. This condition often is confused with other spinal anomalies such as type I congenital kyphosis, sacral agenesis, lumbosacral agenesis, and lumbar agenesis. Faciszewski et al. (
71) gave detailed radiographic and clinical definitions of this condition. Segmental spinal dysgenesis is characterized by severe focal stenosis of the spinal canal at the involved segment and is associated with significant narrowing of the thecal sac and absence of adjacent nerve roots. At the involved level, a ring of bone encircles the posteriorly positioned spinal canal, causing stenosis. The spinal canal is hourglass-shaped with no neurocentral junctions. There is limited potential for enlargement with growth because of the absence of neurocentral junctions, where growth occurs (
Fig. 19-19). No pedicles or spinous or transverse processes are present at this level. Anterior to the bony ring is a fat-filled space. The distal bony anatomy and the spinal canal are usually normal, although spina bifida has been noted in a few cases (
72). Neurologic function can range from normal to complete paraplegia. Associated anomalies are common, and there is a high incidence of neurogenic bladder (
Fig. 19-20).
The etiology of segmental spinal dysgenesis is unknown. The diagnosis can be made on the basis of plain radiographs, but MRI and CT scans and three-dimensional reconstructions are usually needed to fully show the extent of this condition. Tortori-Donati et al. found that the patient’s clinical status correlated with the amount of neural tissue seen on MRI at the level of the lesion (
73). Progressive kyphosis occurs with this condition, and progressive neurologic deterioration was noted by Flynn et al. (
74) and Faciszewski et al. (
71). Early anterior and posterior fusions, with or without
decompression, are recommended. The use of spinal instrumentation is controversial because of the small size of the patient. Hughes et al. (
72) recommended that treatment be directed toward the establishment and maintenance of spinal stability first and toward decompression of the cord secondarily. Bristol et al. recommended rigid spinal immobilization for 12 to 18 months to allow growth and development before spinal fusion (
75).