Types of biopsies
Advantages
Disadvantages
Closed
1. Core needle or fine need aspiration
2. Image-guided percutaneous biopsy
1. Inexpensive
2. No delay in treatment
3. Fewer operative risks
4. Identify by tattooing site of biopsy
1. Insufficient sample (up to 35 %)
2. Difficulty in performing sophisticated histogenic studies
3. Hematoma
4. Bone tumors are difficult to sample
5. Need experienced cytopathologist
Open
1. Incisional
2. Excisional
1. More material for pathology
2. Able to obtain absolute hemostasis
1. Inappropriate placement
2. Infection
3. Dissemination of tumor
4. Cost
5. Logistics of operating time
6. Delay in initiation of neoadjuvant treatment
Open biopsy procedures mitigate some of the potential disadvantages of closed biopsies. Adequacy of specimen can be assured at the time of surgery with frozen section, and therefore, the surgeon can be certain an accurate diagnosis can be made. However, open biopsies must be planned keeping future definitive surgical procedures in mind. Placement of the biopsy incision can facilitate a future successful oncologic resection (see Figs. 6.1 and 6.2). Important considerations of the open biopsy are listed in Table 6.2. Open biopsies do potentially expose a greater area of tissue to tumor contamination and usually do not have the benefit of sophisticated image guidance to direct sampling toward specific areas of the tumor.
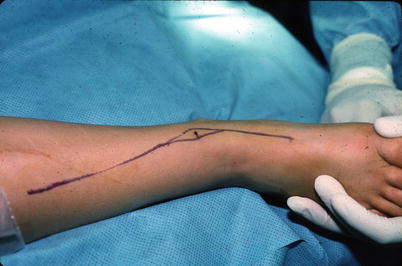
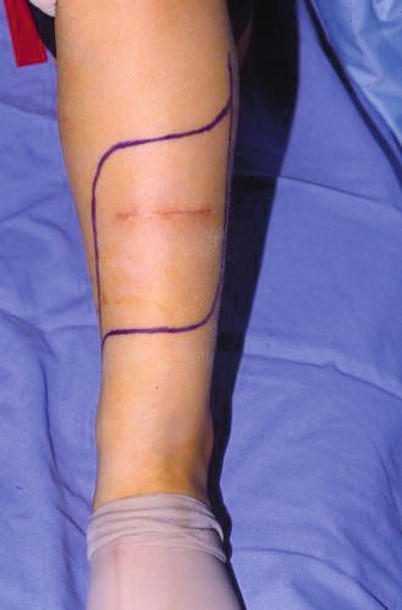

Fig. 6.1
An appropriately placed biopsy tract about the distal tibia in line with subsequent planned excision

Fig. 6.2
An inappropriately placed transverse open biopsy and necessary resection for flap coverage
Table 6.2
Principles of open biopsy
1. Biopsy site needs to be excised with resection |
2. Need to know definitive surgical approach |
3. Stay within a single compartment |
4. Avoid neurovascular structures |
5. Strict hemostasis |
6. No transverse incisions (except along the clavicle or ribs) |
7. Use caution around limb girdles |
Ultimately, both closed and open biopsies can reliably yield diagnostic tissue to guide treatment. The decision between the two is usually heavily influenced by institutional practice patterns, and no studies have shown a clear benefit for one approach over the other.
Amputation
Limb Sacrificing
Amputation is the removal of the affected limb. Resection level is an important consideration. The margin of surgical resection must be planned before surgery based upon preoperative imaging studies, and the planned function of the remaining limb should be known. Ideally, a multidisciplinary team assists with the surgical, functional, and social concerns prior to the procedure. The level of resection is defined by a combination of adequate bony resection to remove the lesion with an appropriate margin as well as allowing an adequate soft tissue envelope to cover the residual limb.
Although many patients opt for limb-preserving surgery, amputation continues to be a viable option for select lesions, particularly if recurrent or locally advanced. In fact, a successful amputation may allow the patient to participate in activities that would otherwise not be possible with traditional reconstructions used in limb salvage. In some circumstances, the aggressiveness of the lesion or the extent of surgical excision precludes limb-sparing options and mandates amputation. Relative indications for amputation include encasement of major neurovascular structures and inability to perform successful vascular bypass, pathologic fracture with hematoma violating compartment boundaries, severe infection, immature skeletal age with predicted leg-length discrepancy >8 cm, extensive soft tissue involvement, and joint contamination.
Limb Sparing
With the advances in preoperative imaging, improved understanding of the biologic behavior of bone lesions, and improvements in implant design and biologic materials, amputation has had fewer indications. Most patients with both benign and malignant lesions are candidates for limb salvage. That said, the surgeon must understand the disease process in order to plan a surgical resection that removes the lesion adequately and maintains function of the limb. Ideally, limb salvage should not delay adjuvant therapy, have rates of local recurrence and overall survival no worse than amputation, and provide enduring reconstruction options that maintain a functional limb equal or superior to that which would be achieved with amputation.
Surgical Margins
It is helpful to understand the principles of limb-sparing resection. Fundamentally, the extent of surgical treatment of bone lesions can be classified by the margin achieved. The surgical margin is classified as intralesional, marginal, wide, or radical by its relationship to the lesion and its “compartment” that it resides within (Table 6.3). Oftentimes the surgeon may be required to use more than one plane of dissection in order to save the limb. In this case, the surgeon may describe the various margins in relation to all of its anatomic boundaries or by its narrowest plane of dissection. The ultimate oncological margin of the procedure is defined by the closest margin achieved.
Table 6.3
Classification of surgical margins
Intralesional excision | Plane of dissection passes within the lesion. Pseudocapsule of the tumor is entered (e.g., curettage) |
Marginal excision | Pseudocapsule of the tumor is not entered. Plane of dissection passes through the reactive zone. Microscopic disease may be left behind |
Wide excision | Plane of dissection passes through a cuff of normal tissue |
Radical excision | Removal of entire compartment of origin; complete extirpation of an entire bone |
Intralesional Excision
Nonmalignant tumors and select low-grade malignancies (e.g., low-grade chondrosarcoma of the extremities) may be treated with intralesional excision. By definition, intralesional excision has a plane of dissection that directly enters the lesion, violating its pseudocapsule and removing the pathology. Intralesional excision can be used for juxta-articular lesions as well as metadiaphyseal lesions. Benign spine and pelvic lesions are also amendable to intralesional procedures.
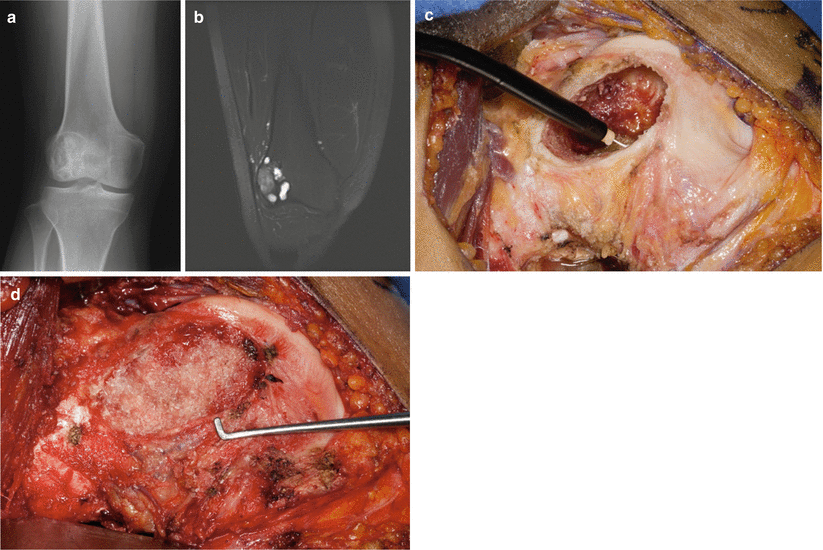
Meticulous dissection down to the bone is essential for adequate visualization and exposure for proper resection. Intraoperative imaging is often useful for localization and extent of the lesion. The cortical bone is then opened with the use of either a drill and osteotome or pencil-tip burr. The cortical window should be made in a round or oval fashion and should be large enough to completely exteriorize the lesion. Squared edges should be avoided because of the stress raiser effects and difficulty with full exteriorization of the lesion, which is needed for complete removal of the pathology. The bone lesion is then removed with the use of surgical instruments like curettes with care taken to avoid contamination into the approach tissues.
Once all gross tumor is removed, the cavity is usually treated with the use of an adjuvant to reduce the risk of recurrence. Adjuvants include a combination of chemical, thermal, or mechanical and are useful in treating microscopic cells that are thought to escape the initial curettage (Table 6.4). It is thought that microscopic cells that are left behind increase the likelihood of local failure.
Table 6.4
Types of adjuvants that are used to extend the margins of an intralesional excision
Chemical |
1. Hydrogen peroxide |
2. Distilled water |
3. Alcohol |
4. Phenol |
Mechanical |
1. High-speed burr |
2. Curettage |
Thermal |
1. Argon beam coagulator |
2. Electrocautery |
3. Polymethylmethacrylate (PMMA) |
4. Liquid nitrogen |
5. Cryoprobes |
Mechanical Adjuvants
Mechanical adjuvant treats microscopic disease by essential debridement. High-speed burring is one of the most commonly employed examples of a mechanical adjunct. Not only does the high-speed burr debride the walls of the cavity, the heat generated during the procedure may also add a thermal component as well. Complete exteriorization of the lesion is important in order to be able to access the entire cavity.
Chemical Adjuvants
Various chemicals have been employed as adjuvants in the setting of intralesional curettage. The ability of liquid to permeate into the endosteal scaffolding and reach places that may be inaccessible for mechanical treatments is the main advantage of chemical adjuvants. However, the surgeon must remain cautious as some chemicals, such as phenol, are caustic and can cause damage to the surrounding bone, soft tissue, and vital structures (e.g., nerves, cartilage, joints, and muscle). Even less toxic chemical applicators (e.g., hydrogen peroxide) are messy and can be difficult to contain. Therefore, it is important that the intralesional procedure assures that the treated cavity is contained prior to the use of these agents.
Thermal Adjuvants
Thermal adjuvants are also used to help in the treatment of these lesions. As with its chemical counterparts, thermal applications have the ability to affect surrounding structures, and the surgeon must be cognizant of the surgical “topography” prior to using these agents. Some surgeons believe that polymethyl methacrylate (PMMA), bone cement, is useful in this capacity. The exothermic polymerization of the bone cement increases the temperature of the lesion and theoretically can cause necrosis of a layer of endosteal cells hopefully eradicating lesional tissue. Additionally, an advantage of PMMA is its structural properties which primarily include its compressive strength making it a useful tool when managing juxta-articular lesion. It also has the advantage of allowing the surgeon to detect recurrence because the PMMA provides a permanent contrast for future surveillance. Although bone cement has certain advantages, it too has the potential for inadvertent extravasation if the lesion is uncontained. Additionally, there is evidence that the heat can damage articular cartilage when used close to the subchondral bone.
Cautery can also be used as a thermal agent. The argon beam coagulator is the classic example of this type of use. Often referred to as the argon “laser,” the cautery is not a laser, but rather makes use of a supply of argon gas to effectively treat the lesional surface. An alternative to the argon beam coagulator is using a standard electrocautery. By turning the electrocautery up and using it in a fulgurate setting, the surgeon can thermally treat the lesion in a similar fashion as the argon beam coagulator. These have the advantage of giving the surgeon more control over treating the lesion. However, as with mechanical adjuvants, full exteriorization is necessary to access the cavity. Often it may be extremely difficult if not impossible for the surgeon to treat the entirety of the lesion completely. If a heat-based thermal adjuvant is used, it should not follow the use of any flammable chemical adjuvants. Liquid nitrogen or the use of cryoprobes can also be used as a thermal adjuvant. No study has established the superiority of one thermal adjuvant over the other, and the selection is usually influenced by surgeon preference and institutional practice patterns.
Most often, more than one if not all types of adjuvants are employed with extended intralesional surgical procedures. In order to prevent contamination of debris that occurs with high-speed burring, the lesion is often treated with chemical or thermal adjuvants first. Moreover, argon coagulation and some chemical adjuvants (e.g., phenol) stain the walls of the cavity and can provide the surgeon with a guide to a very controlled and complete treatment. The burr can then be used to remove this layer leaving no doubt that the entire lesion was treated. The cycle can then be repeated, effectively extending the boundary, or margin, of the lesion into the normal bone.
Once all gross disease has been removed and microscopic disease has been treated with the use of adjuvants, the remaining cavity will then have to be addressed. Occasionally, small lesions or those that are in limited weight-bearing areas may be left to fill in and heal on their own. However, the majority will require filling with a bone substitute (Fig. 6.3). Bone cement (PMMA) has utility as both an adjuvant and a bone filler. It provides immediate strength, prevents articular collapse, and provides a permanent contrast to identify recurrence (Fig. 6.4). Moreover, it is useful as a biological carrier for local antibiotic delivery. However, its use around joints must be judiciously utilized to avoid extravasation and can make future surgical procedures more difficult.