Fig. 12.1
Pain circle describing migration of pain and the multifaceted nature of pain in athletes’ groin. The majority of patients may have initially (1) insertion tendonitis of adductors, (2) sports hernia, i.e., weakness of the posterior wall of inguinal canal, and (3) pubic bone marrow edema, i.e., osteitis pubis. The chronic pain may change place and also circle from adductors to pubic symphysis
To achieve exact diagnosis, an experienced multidisciplinary approach is required including a physiotherapist, orthopedic surgeon, and a hernia surgeon as key members. Sometimes a multidisciplinary examination may reveal more than one of the above mentioned reasons for chronic groin pain [4, 5]. Surgical approach includes various types of open techniques with or without mesh, laparoscopic transabdominal pre-peritoneal (TAPP) or total extraperitoneal (TEP) techniques. The results of the operative treatment from single centers are reported to be good to excellent in between 70 and 90 % of patients [5–10].
The aim of this chapter is to focus on the pathophysiology, diagnosis, and endoscopic treatment of sports hernia.
Pathophysiology
No exact pathophysiological mechanism for chronic pain has so far been identified in SH or AP. The most common theories include adductor and/or rectus abdominis muscle tendinopathies, hip hyperextension injuries, or disruption of the posterior wall of inguinal canal [6, 7, 9, 10]. A tear of the abdominal wall in the posterior inguinal canal or conjoined tendon (tendinopathy), with or without bulging like a hernia, was originally suggested to be typical for a SH [4, 7, 9]. The tissue damage or disruption of the posterior wall is analogous to an incipient direct inguinal hernia (with or without bulge). To date, there are no histological, imaging, or operative studies which confirm the causal relationship between microscopic and/or macroscopic tissue injury to chronic pain in SH.
Diagnosis of Sports Hernia
Patient History
The location of pain is usually rostral to the inguinal ligament in the deep inguinal ring with or without tenderness over the pubic symphysis. Pain is often dull and diffuse radiating to the perineum and inner thigh or across the midline. Most athletes cannot determine how or when the groin pain began. Pain may also migrate and change place from one side to another (Fig. 12.1). The strong pull of the adductors or rectus muscle may create a shearing force across the hemipelvis and symphysis resulting in periosteal inflammation and, finally, injury of the symphysis pubis.
Imaging
Plain X-ray, isotope bone scan, ultrasound (US), magnetic resonance imaging (MRI) or herniography may sometimes help to detect the exact cause of pain [1–3]. Especially MRI is recommended to exclude other soft tissue injuries in the groin area (Fig. 12.2). Osteitis pubis, with its associated BME, can be identified on MRI [5].
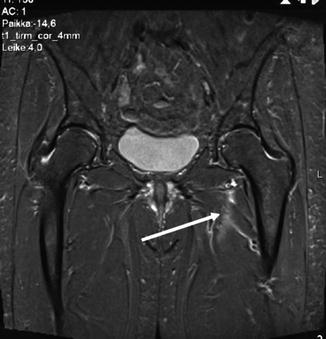

Fig. 12.2
Differential diagnosis of sports hernia from other causes of chronic groin pain is essential. Magnetic resonance imaging is often used to diagnose many non-operatively treated injuries. This 25-year-old soccer player with chronic groin pain had rupture of the obturator externus muscle (arrow), which became symptomless after 8 weeks follow-up
Clinical Examination
A thorough clinical examination of the inguinal area and scrotum should be performed to exclude an inguinal hernia or a scrotal disorder. The attachment of the tendons of the rectus abdominis and adductor muscles should be examined for pain. The hip and back should be thoroughly examined including restriction of movement, pain and nerve functions (including the inguinal region). The clinical findings, both negative and positive, should be thoroughly reported.
All imaging studies are surprisingly often negative and the only clinical sign is deep pain at palpation in the area of the pubic tubercle. In such cases SH can be suspected. Diagnosis of SH can only be set in patients having a typical history and having a suspected posterior inguinal wall deficiency on careful clinical examination. MRI maybe negative or indicate only some irritation or pubic symphysis (BME) in SH.
Differential Diagnosis
The combination of complex anatomy in the groin area, variability of presentation, and the nonspecific nature of the signs and symptoms make sometimes the differential diagnostic process problematic [11].
Osteitis Pubis
Osteitis pubis is one differential diagnosis to SH. It is characterized by diffuse pain, inflammation, and bone changes in the pubic symphysis. BME being a reminiscent of osteoarthritis is the hallmark on MRI findings of osteitis pubis [12–15]. MRI is a highly sensitive modality to depict tissue water and inflammation. It has been reported that BME in MRI does not give any additional information during an active training period compared to a training-free interval in asymptomatic heavy-training athletes [16–18]. Thus, the relevance of BME in the symphysis pubis is of questionable importance when operative treatment is planned for SH and AP [16].
Femoro-Acetabular Impingement Syndrome
Another important differential diagnosis is femoro-acetabular impingement (FAI). Flexion, internal rotation, and adduction of hip joint are typical movements that are painful in patients with FAI.
It has recently been stressed that in some patients with AP both hip and pubic regions are concurrently injured. When surgery is addressed in these patients for only one of the two pathologies, either the AP or the FAI, the outcome will be suboptimal. Surgical management of both disorders concurrently, or in a staged manner, may lead to improved postoperative outcome, scoring, and an unrestricted return to sport activity [19].
Surgical Approach of Sports Hernia/Athletic Pubalgia
Treatment of SH and/or AP aims toward its specific pathology. First line management includes rest, muscle strengthening and stretching exercises, physiotherapy, anti-inflammatory analgesics, local anesthetic, and/or corticosteroid injections and, in resistant cases, operative management [1, 2].
Surgery should be undertaken by surgeons with vast experience in this field. A recommendation is to evaluate these patients in a “multi-specialist sports team” including a general surgeon, an orthopedic surgeon, and a physiotherapist. Educational programs are encouraged including mentorship followed by a certification. Competence has become a major concern and an essential requirement in the surgical community.
There is no evidence-based consensus available on indications for operative treatment of AP [1]. Both open and laparoscopic repairs are good and seem to result in equivalent results. The latter may allow earlier return to full sport [5, 8, 10]. Comparative or randomized studies between open and laparoscopic procedures are lacking. Endoscopic repair of SH, particularly in athletes, has many theoretical advantages. The posterior position of the mesh, that will include the conjoint tendon and pubic bone, creates theoretically a stronger support than a conventional anterior hernioplasty (Fig. 12.3).
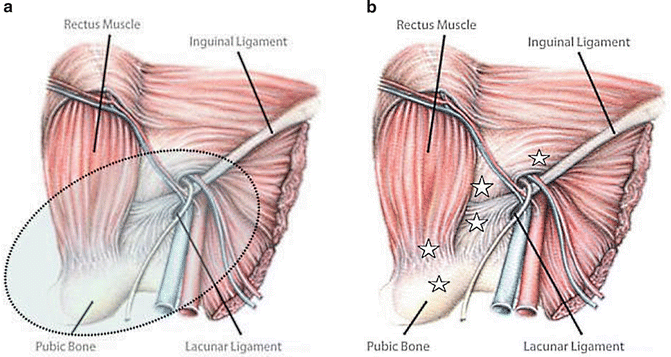

Fig. 12.3
Both in TAPP and TEP procedures the mesh is placed in the pre-peritoneal retro-pubic space (light blue area in a), and it covers most of the injured areas (white star) in athletic pubalgia (b). Groin disruption can be on the periosteum of pubic symphysis, posterior wall of the inguinal canal, pubic insertion of rectus abdominis muscle and inguinal ligament, as well as on the internal ring. All these areas are marked with stars
Generally, postoperative pain and wound complications are less frequent after laparoscopic surgery than in open surgery. There is a huge experience in inguinal hernia surgery on advantages and disadvantages of different techniques. The endoscopic techniques seem superior when it comes to the sensation of a foreign body in the groin or chronic pain in mesh repairs [20, 21]. The open techniques include dissection in an area where the three inguinal nerves are found (the ilio-inguinal, the iliohypogastric, and the genital branch of the genitofemoral nerve) and the surgeon has to take notice of them. Harm of these nerves might result in restrictions and chronic pain frequently reported on after inguinal hernia surgery [22]. The consideration for nerve problems is less in the endoscopic techniques, especially when following the right planes for dissection and avoiding fixation [23].
The TEP technique has the advantage of being performed totally pre-peritoneal, thereby not entering the abdominal cavity. In TAPP you enter the cavity, which is a potential risk of harming intra-abdominal structures. However, the TAPP approach does allow the possibility to perform a diagnostic laparoscopy which might be indicated especially in female where differential diagnosis intra-abdominally might be considered. In the TEP approach, a pocket is dissected where a mesh can be placed without fixation or by the use of atraumatic glue [23]. In TAPP, some type of fixation of the mesh, usually by spiral tacks, is common and sometimes tacks are also used to cover the mesh with peritoneum. The pre-peritoneal endoscopic technique results in less dominant scars than the open techniques.
A novel, open, minimal suture repair technique, restoring the transverse fascia, may be a promising technique to repair sportsman’s groin [6]. Whether the endoscopic or the open technique is better than the other (concerning relief of pain) is now tested in a randomized controlled trial incorporating an international specialized team of surgeons experienced in both techniques (Clinical Trials NCT01876342).
Transabdominal Pre-peritoneal Technique
All endoscopic SH operations are performed under general anesthesia [21]. Patients are placed in a supine position. The patient is catheterized after induction of anesthesia. In TAPP technique, pneumoperitoneum is established, preferably using an open access technique below the umbilicus to minimize the risk of blind puncture of intra-abdominal structures. A 10-mm port is placed with 30° camera used for initial visualization of the peritoneal cavity. Two 5-mm working ports are introduced for dissection. The peritoneum is incised well above the internal ring and the peritoneum is dissected 5 cm beyond the margin of the groin defect medially exposing the Cooper’s ligament and laterally/dorsally well exposing the triangle of doom (Fig. 12.4). An appropriate-sized (usually 10 cm × 15 cm) polypropylene mesh is chosen, allowing at least 5-cm overlap on the injury of the posterior wall. The mesh is unrolled in the pre-peritoneal space and fixed onto the abdominal wall with helical tacks, sutures or tissue glue including the posterior pubis and Cooper’s ligament [23]. Re-peritonealization is usually performed by continuous intra-corporeal suturing of the peritoneal flaps with 3–0 polyglactin suture or using tissue glue or tacks. Conventional non-steroidal anti-inflammatory drugs (NSAID) or paracetamol are used for postoperative pain relief. The operation is performed as day case surgery and follow-up is performed according to local protocol.
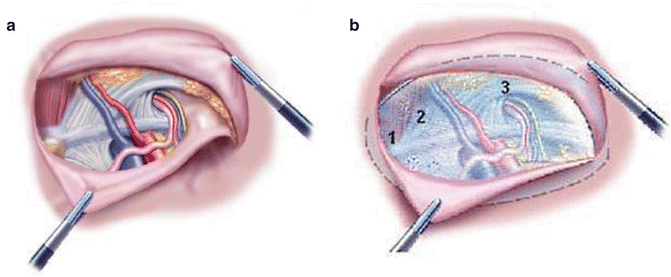

Fig. 12.4
In transabdominal pre-peritoneal technique (TAPP) mesh is introduced into the pre-peritoneal space through the abdominal cavity by opening the peritoneum (a). Mesh is tacked with titanium clips or tissue glue and covers pubic symphysis (1), posterior wall of the inguinal canal (2), pubic insertion of rectus abdominis muscle and inguinal ligament, as well as internal ring (3) (b)
Laparoscopic mesh implantation in TAPP usually follows the current principle of inguinal hernia surgery and is based on the physiological principle of diffusing the intra-abdominal pressure on the whole area of the implanted mesh in the disrupted groin area (Fig. 12.4). This ensures immediate firm fixation of the mesh to the abdominal wall [21]. The pre-peritoneal mesh covers the most vulnerable and injured areas at the pubic symphysis complex, and also supports firmly the posterior wall of the inguinal canal. The tissue ingrowth is expected to be more extensive than a mesh placed directly on the peritoneal surface.
TAPP technique was the initial endoscopic treatment of SH reported by UK surgeons [24]. According to original reports this technique “appeared as effective as conventional repair for sporting injuries and merits further evaluation as a technique to permit early return to activity” [24]. Soon after that, many more athletes were operated using TAPP technique [8, 10, 25–29].
Totally Extraperitoneal Technique
Area of maximal pain can preferably be marked preoperatively on the groin skin which helps to place the retro-pubic mesh in an optimal position (Fig. 12.5). In TEP, the retro-pubic pre-peritoneal space is approached through a small sub-umbilical skin incision, followed by the entrance through the anterior rectus fascia and a muscle split, entering the pre-peritoneal facial space using a blunt 10 mm port on the affected side. The use of balloon dilatation is optional. The surgeon can also open the pre-peritoneal space by using the camera together with gas insufflation which is convenient and cheap (Fig. 12.5). After dissection described above, the retro-pubic area is covered with a 10 cm × 15 cm low weight polypropylene mesh, preferably using no-fixation or tissue glue [23, 30]. Meshes are sometimes placed bilaterally even if the symptoms are unilateral, because scarring of the pre-peritoneal space would most likely prevent a later operation of the non-affected side. There is, however, no consensus presented in the literature for bilateral operation.
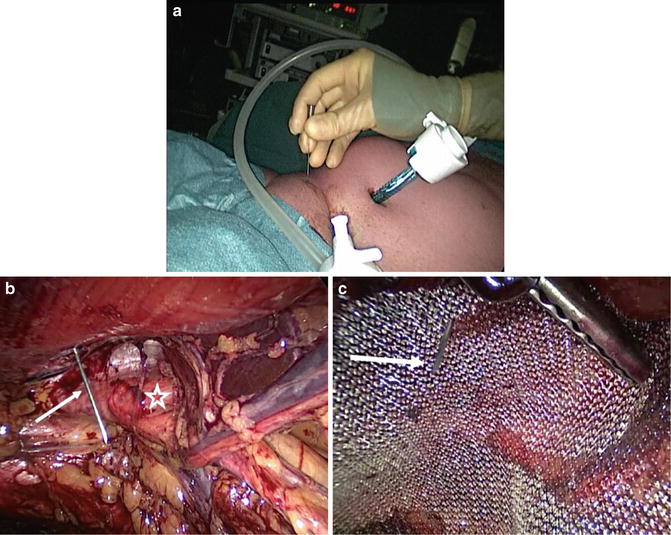

Fig. 12.5
Positioning of the mesh in the TEP technique. Preoperatively, a maximum pain area can be skin-marked (a) and during operation an injection needle (white arrow) is introduced into retro-pubic space (b, c). This patient had disruption of the posterior wall of the inguinal canal (white star)
Results of TAPP and TEP
Altogether 675 laparoscopic operations for AP, 504 TAPP, and 171 TEP are reported in the literature (Table 12.1). Early results indicate that athletes return to their sports activities in 70–90 % already after 3–4 weeks after both TAPP and TEP. In the report by Ingoldby et al., 7 out of 14 patients who had a TAPP repair denied pain postoperatively. Training was resumed within 4 weeks for 9 out of 14 patients who had an open repair and 13 out of 14 who underwent TAPP. Full contact training was restarted at a median of 5 weeks (range 1–6) for open and 3 weeks (range 1–9) for TAPP (p < 0.05) [24, 28]. Others have confirmed these results after endoscopic surgery. Nearly all athletes (90 %) were able to return to full, unrestricted athletic activity within 4 weeks or less. Overall long-term satisfaction of TAPP was high and long-term follow-up revealed no or very few adverse sequelae or recurrent symptoms [8, 26].
Table 12.1
Endoscopic treatment of sports hernia—a review of the literature
Author and year | Number of athletes | Follow-up | Return to sports | Recovery (%) | Complications (N) |
|---|---|---|---|---|---|
Transabdominal (TAPP)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |||||




