CHAPTER 70 Surgical Treatment of Cervical Myelopathy
INDICATIONS
Surgical treatment is indicated for patients with moderate to severe myelopathic signs and symptoms such as functional weakness, loss of dexterity, or gait abnormality and who are otherwise sufficiently healthy to withstand the physiological stress of anesthesia and surgery. There is no consensus regarding the optimal treatment of mild forms of myelopathy such as patients with mild sensory alteration and hyperreflexia. Early surgical intervention can be considered a reasonable option for patients with radiographic evidence of static cord compression and clinical signs and symptoms of early myelopathy, with the goal of preserving neurological function and limiting the risk of future spinal cord injury. A period of nonoperative observation is an option for patients with clinical signs of early myelopathy but no significant functional compromise. In the absence of overt clinical signs or symptoms or myelopathy, surgical treatment of radiographic findings such as mild cord compression on magnetic resonance imaging (MRI) should be entered into with caution, given evidence that such findings may be present in as many as 16% of asymptomatic subjects under 64 years of age and in 26% aged 65 years and older.1
Mild, moderate, or severe forms of myelopathy remain clinical impressions without clearly defined criteria. Clinical studies have employed various scales including the Nurick grading system and the Japanese Orthopaedic Association system. Benzel’s modification of the JOA scale (mJOA) is more applicable to Western populations. (These classifications systems are outlined in previous chapters.) Using this system, an mJOA score <12 can be considered moderate myelopathy, while severe myelopathy is reflected by a score <7 (maximum score=18).2
ANTERIOR SURGERY
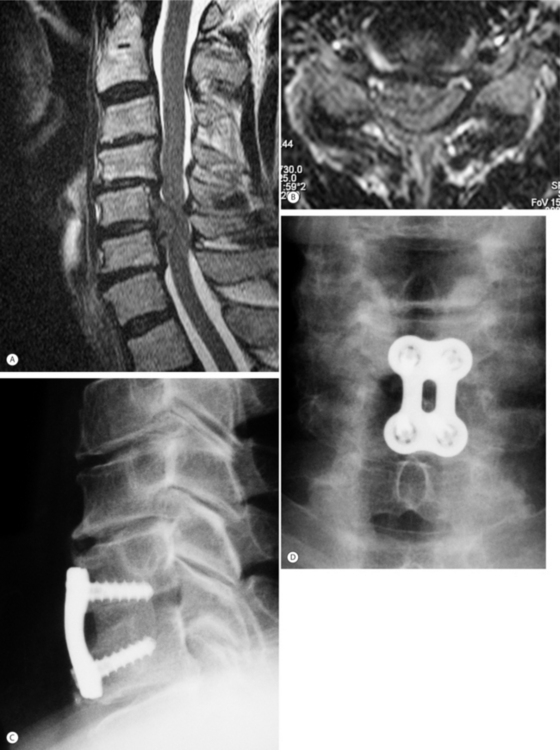
An anterior surgical approach is an option when spinal cord compression results from anterior pathology such as herniated disc material, endplate or uncinate process osteophytes, or ossification of the posterior longitudinal ligament (OPLL). The anterior approach is ideal for compression limited in extent to one or two spinal levels, and may provide superior results over posterior approaches by allowing direct excision of the offending pathology (Fig. 70.1). Although a posterior approach can achieve indirect decompression of the spinal cord in many patients, the surgery often must be extended over a greater number of levels. In patients with significant cervical kyphosis and anterior cord compression, a posterior-only approach will not achieve sufficient decompression, and in most cases anterior surgery is required.
For the majority of anterior surgical procedures, we favor use of a standard operating table. Patients with severe myelopathy or canal stenosis should undergo anesthetic induction on the operating table to minimize manipulation while the patient is unconscious. Fiberoptic intubation is preferred to limit extension of the neck. Neurophysiologic spinal cord monitoring employing both sensory and motor evoked potentials is a potentially useful adjunct. A baseline measurement should be obtained prior to anesthetic induction and patient positioning. The patient is placed supine in 15–30° reverse Trendelenburg to reduce intraoperative bleeding. For one- to two-level discectomy or a one-level corpectomy, a 4 cm transverse skin incision is used from the midline to the medial border of the sternocleidomastoid muscle. For surgery involving three or more levels a transverse incision can be used but for difficult exposure, partial or complete transaction of the omohyoid muscle will improve exposure. A longer oblique incision is an alternative used along the medial border of the sternocleidomastoid muscle but it is less cosmetic. To reduce risk of injury to the recurrent laryngeal nerve, some surgeons prefer a left-sided incision and approach due to the more consistent anatomic location of the nerve on the left side within the tracheoesophageal groove. Superficial dissection consists of incision through skin, subcutaneous tissue, and platysma. Deep dissection then proceeds through a natural anatomic plane between the trachea and esophagus medially and the sternocleidomastoid muscle and the carotid sheath neurovascular bundle containing the internal jugular vein, vagus nerve, and carotid artery laterally. Gentle retraction of the trachea and esophagus medially and blunt dissection through the intervening middle layers of the deep cervical fascia reveals the longus colli muscles and prevertebral fascia overlying the anterior cervical spine. Subperiosteal elevation of this muscle and fascia layer allows placement of self-retaining retractors and sufficient visualization to proceed with decompression. A simple intraoperative maneuver once the retractors are in place and opened is to deflate and then reinflate the endotracheal cuff to more evenly distribute pressures within the endolarynx and possibly minimize excessive pressure on branches of the recurrent laryngeal nerve, especially for a right-sided approach.
Anterior decompression
Corpectomy
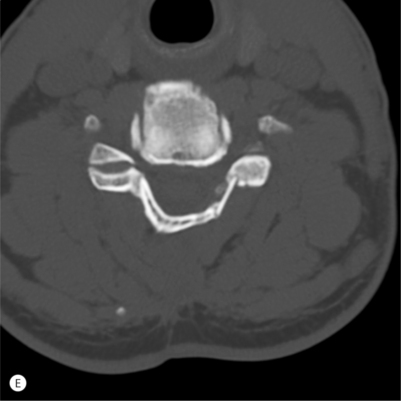
In cases of OPLL, an attempt should be made to remove any ossified tissue contributing to compression of the spinal cord or nerve roots. In cases of severe compression, localized areas of dural erosion may be present when overlying tissue is removed. Even with an apparently intact subarachnoid membrane, postoperative cerebrospinal fluid (CSF) leakage and delayed formation of durocutaneous fistulas may occur, and consideration should be given to patching such defects with muscle or fascia and placing a lumbar subarachnoid shunt.3 Radiographic signs of dural penetration by the ossified posterior ligament have been suggested, including the ‘C’ sign and single-layer sign on plain radiographs or the double-layer sign on computed tomography.4 When these signs are present, an option is to avoid complete removal of overlying ossified tissue and leave free-floating patches attached to small areas of suspected dural penetration. This is accomplished by releasing the posterior longitudinal ligament laterally and allowing the thinned-out remaining ossified ligament to float forward, decompressing indirectly the spinal canal.
An oblique corpectomy is an alternative technique that can be performed to achieve anterior decompression without the need for concomitant fusion. Proponents of this procedure suggest ideal candidates for this procedure as having asymmetric cord compression from spondylotic bars that are predominantly unilateral in location.5 The intervening disc space must be dehydrated and collapsed to limit the risk of postoperative instability. Bilateral foraminal stenosis is a contraindication.
With this technique, the rate of significant complications approaches 30% and therefore limits its potential application. Horner’s syndrome occurs transiently in up to 57% of patients but can be permanent in up to 9%.6 A prospective study of 26 patients treated by this technique resulted in 76.9% good to excellent results with 84.6% improvement in any preoperative radicular symptoms.5
Anterior reconstruction
A problem with both autologous and allograft bone reconstruction is the need to place these relatively straight grafts in an inherently less stable anterior position to avoid spinal cord impingement. Precontoured titanium mesh cages are available for longer reconstructions that allow more central positioning on endplates while preserving a degree of lordosis.7
Anterior plating
Supplemental cervical plate and screw fixation is routinely recommended following multilevel anterior discectomies and corpectomy procedures. Plate fixation for single-level discectomy and fusion obviates the use of postoperative cervical brace but there is no clear evidence that it is superior to in situ fusion. If patient bone quality allows adequate fixation strength, instrumentation provides immediate stability, reducing early postoperative pain and in many cases obviating the need for weeks of postoperative hard collar or halo vest immobilization. Long-term outcome studies have demonstrated significantly improved fusion rates and decreased rates of graft-related complications associated with use of anterior plates.8,9 In the absence of instrumentation, 50% of patients undergoing single- or two-level corpectomy experience localized kyphosis of 10° or more as a result of graft subsidence.10 Preservation of cortical endplates can minimize such subsidence but requires the use of anterior plates to prevent graft extrusion and may increase the risk of pseudoarthrosis.
POSTERIOR SURGERY
Laminectomy
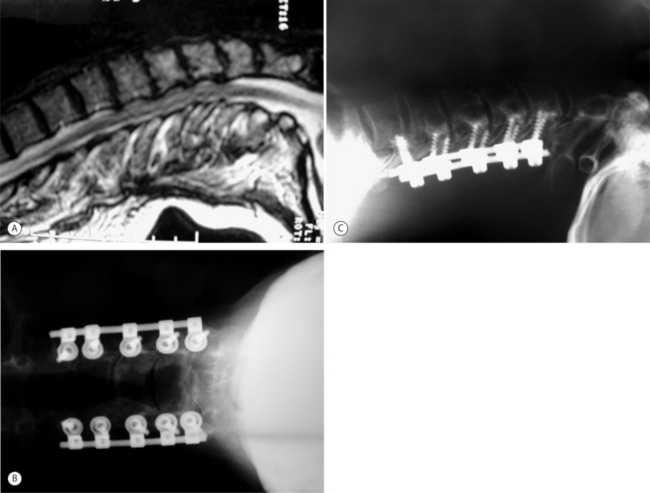
Cervical laminectomy has been associated with a 20% incidence of late postoperative kyphotic deformity in the adult patient.11 Preoperative kyphosis is therefore a contraindication for laminectomy unless concomitant anterior reconstruction can restore at least neutral sagittal alignment. In patients with preoperative neutral or lordotic sagittal alignment, laminectomy is technically straightforward and yields typically good results in conjunction with a posterior fusion procedure (Fig. 70.2). Ideal candidates have multilevel stenosis that would be difficult to reconstruct following anterior decompression. Patients with extensive OPLL are also candidates to avoid the risk of dural tears with anterior decompression. The operation is also well suited for elderly and debilitated patients who may not tolerate a longer anterior procedure with potential for greater blood loss. Patients with significant calcification of the ligamentum flavum causing posterior cord compression are also good candidates.
Technique
The posterior cervical spine is exposed to include all involved levels. Care is taken to avoid injury to the facet capsules of any levels that are not to be surgically fused. If an instrumented fusion is planned, then we recommend preparing screw holes for all implants prior to performing the actual laminectomy in order to minimize the risk of iatrogenic cord injury during instrumentation. The interspinous ligaments are resected proximal and distal to the levels to be decompressed. The laminectomy margins are defined anatomically by the longitudinal border between the lateral masses and the laminar bone. A high-speed burr is used to sequentially divide the laminar bone along this border on one side. Although this step is not typically associated with significant blood loss, an extensive epidural plexus of veins is concentrated near the junction of the lamina and lateral mass, and excessively lateral burring of the troughs can lead to substantial hemorrhage. We recommend placement of the troughs 2 mm medial to the junction of the lamina and lateral mass to minimize this risk. A Kerrison rongeur can be used to complete resection of the inner cortical bone. The contralateral laminar bone is then divided in similar fashion. With division of the ligamentum flavum, the resected laminar bone can be elevated sequentially and removed en masse, completing the laminectomy. Because of the increased incidence of postoperative C5 and C6 root palsy, a concomitant C5 and C6 foraminotomy is routinely prophylactically performed.
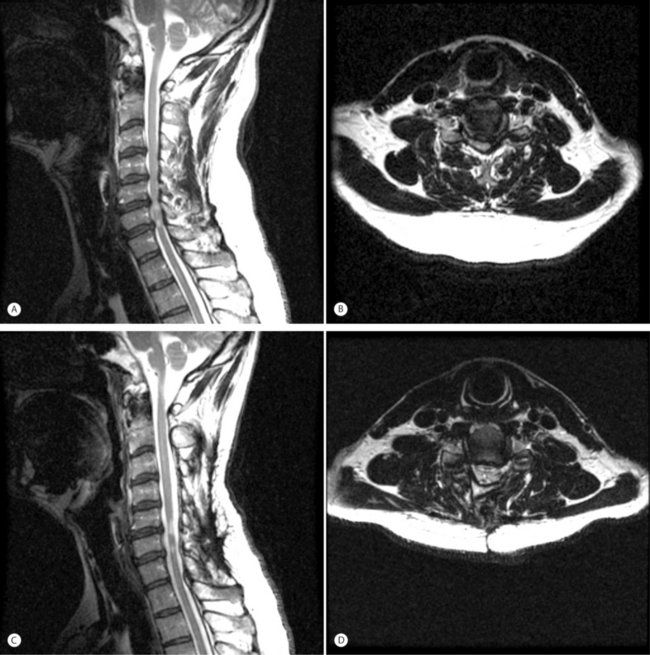
Laminoplasty
Development of cervical laminoplasty was pioneered by Japanese surgeons for treatment of OPLL and first reported in the English language by Hirabayashi et al. in 1983.12 Extensive anterior decompression and reconstruction procedures were associated with high failure rates and motivated development of a posterior approach that was less destabilizing and did not require complex reconstruction techniques. The surgical indications were subsequently expanded successfully to include patients with more generic spondylotic myelopathy as well. Laminoplasty results in lower rates of postoperative kyphosis compared to laminectomy without fusion. Elevating the laminar bone on a hinge expands the sagittal diameter of the spinal canal, allowing the spinal cord to migrate posteriorly, accomplishing an indirect decompression (Fig. 70.3). In patients with OPLL, postoperative imaging demonstrates posterior cord translation of up to 6 mm accompanied by increased transverse cord area, both of which correlate with improvements in myelopathy.13 Following the original description of the procedure by Hirabayashi et al., now referred to as ‘open-door’ laminoplasty, several modifications including ‘French-door’ and ‘skip’ laminoplasty have been reported and are currently being practiced.14–16
Prior to surgery, careful assessment of a lateral radiograph in the neutral position must be made. Kyphotic sagittal alignment is considered by many to be a contraindication for laminoplasty. Either neutral or lordotic alignment is acceptable, although in patients with excessive lordosis there may be a risk of overexpansion of the canal diameter. Due to potentially increased risk of C5 palsy in patients with severe lordosis and excessive posterior cord migration, limiting the amount of laminar elevation to approximately 12 mm may minimize the degree of neural stretch. Preoperative spondylolisthesis is not considered a contraindication for laminoplasty, as 85% of spondylolisthesis cases have been observed to resolve or improve within 1 year following surgery.17 In terms of prognosis, however, posterior spondylolisthesis may be associated with lower rates of postoperative neurological recovery.
Technique
Sutures can be used to maintain the hinged laminae in the open position, or spacers made of various materials, including spinous process bone, allograft bone, or hydroxyapatite, or miniature titanium plates can be utilized to block open the laminae. When fashioning allograft spacers to support the opened posterior arch, an ideal height of 10–15 mm allows adequate cord decompression while at the same time limiting the extent of posterior migration and possibly reducing the risk of C5 root palsy.18
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree