Surgical Management of Bone Tumors in the Foot and Ankle
Hilaree B. Milliron
Joseph A. Favazzo
B. Hudson Berrey
Primary bone tumors, as well as soft tissue tumors, are infrequently found in the foot and ankle. Tumors of soft tissue are seen much more often than those in bone. Benign tumors of bone and soft tissue are not uncommon in the foot and ankle (1); however, malignancies and metastases are exceptionally rare (2,3,4,5 and 6). Unfortunately, when metastatic disease presents in this location, it is most often in the late stages of systemic disease. The rarity of encountering a pedal malignancy puts the less-experienced foot and ankle surgeon at greater risk of complications and at a greater risk of having to face those that are potentially life and limb threatening. In fact, most providers seldom see a malignant tumor in their entire career, which can lead to a lack of recognition when the disease does appear (7,8 and 9).
The biopsy lays the groundwork for all tumor-related surgical management. Its role is critical in the treatment of musculoskeletal neoplasms. While certain tumors may be diagnosed based on radiographic appearance, tissue confirmation of tumor type is crucial prior to lesion resection since there is such wide overlap between numerous diagnoses. Proceeding to a definitive surgical procedure prior to having a diagnosis should be avoided. The risks and consequences of this continuing on to surgical treatment unprepared are well-documented in the foot and ankle literature.
All approaches to the evaluation and treatment of tumors of the foot and ankle must be guided by a single primary principle—local control of the tumor. This is the first and most vital consideration in devising a surgical plan and guides the decision of the tissue to be preserved, the tissue to be removed, and the margins that will be achieved. Maintenance of function, while a paramount consideration in most other surgical endeavors, is of lesser importance. A less than optimal tumor resection with an effort to preserve a degree of function can lead to recurrence of the disease, which can bring about systemic disease and death. Inadequate tumor resection can also lead to additional, more complicated surgeries.
EXAMINATION
A complete history and physical exam are of utmost importance in the initial basic workup of any bone tumor. The chief complaint is most often nonspecific pain or a painful mass. However, an incidental radiographic finding may be the inciting event in the patient that presents with a bone tumor. Age and sex are often crucial in the process of developing a differential diagnosis. The patient’s overall health should certainly be assessed, in addition to the specific area of the lower extremity under consideration. Has the patient had fatigue or weight loss? How large is the mass? Is the mass tender, mobile, or hot to the touch? Are there any significant clinical differences compared with the contralateral lower extremity? Are there any palpable or painful lymph nodes?
Following the history and physical examination, a thorough understanding of the local anatomy involved with the tumor is critical. Radiographs are certainly an indispensible piece of the diagnostic puzzle. For bone lesions, it is estimated that the diagnosis of the tumor type can be made from plain film radiographs 90% of the time. Dormant or benign-appearing lesions require serial radiographs at regular intervals to evaluate for changes in growth and may be disregarded after approximately 2 years with no radiographic changes or increase in pain. However, aggressive lesions warrant immediate initiation of a more aggressive treatment plan. Ancillary imaging modalities are routinely ordered with suspicion of a plain-film tumor or tumor-like lesion. Apart from its cross-sectional imaging capabilities, bone formation, endosteal and periosteal bone surfaces, cortical structure, and calcifications are best visualized with computed tomography (CT). CT-guided biopsy may be performed. Magnetic resonance imaging (MRI) may indicate the biologic nature of the tumor and can help surgical planning by accurately determining the tumor’s extent and boundaries. Nuclear imaging can also be used as a skeletal screening device to detect other bone lesions, as in the case of metabolic disease, multifocal disease (e.g., enchondromatosis), or metastatic disease. Angiography may be helpful in identifying a highly vascular tumor. Ultrasound may be advantageous, but is highly dependent on the skill of the sonographer. Imaging of soft tissue and bone neoplasms is described in much greater detail elsewhere.
Age and plain film radiographs together may perhaps lead the surgeon to the diagnosis. In review, certain bone tumors have a predilection for specific regions. In the skeletally mature patient, epiphyseal lesions are likely giant cell tumors (GCTs), while a chondroblastoma may be suspected in a child. Ewing sarcoma, histiocytosis, and fibrous dysplasia are found in the diaphysis. Nonossifying fibromas, solitary bone cysts, chondromyxoid fibromas, and osteosarcomas are likely to arise in the metaphysis.
A more focused workup is essential if malignancy is, by any means, suspected. New radiographs should be taken when there is any uncertainty. Lab values may be helpful in certain circumstances, such as a complete blood count with differential, erythrocyte sedimentation rate, electrolytes, liver function tests, calcium levels, alkaline phosphatase levels, and lactate dehydrogenase. Although not routinely obtained, blood and urine specimens can be used to rule out multiple myeloma, metastases, Paget disease, and infection. Additionally, further imaging, such as MRI, CT, and technetium bone scanning of the region, should be performed to serve as a complete staging workup for cancer. These imaging modalities allow for the best
surgical preparation. An abdominal, pelvic, and chest CT scan or a positron emission tomography scan should be performed if malignancy is suspected to determine if metastatic disease exists. A biopsy must be carried out as the last step.
surgical preparation. An abdominal, pelvic, and chest CT scan or a positron emission tomography scan should be performed if malignancy is suspected to determine if metastatic disease exists. A biopsy must be carried out as the last step.
Organized tumor treatment should be the perfect picture of teamwork. The surgeon plays one of many roles required in the therapeutic regimen and owes it to the patient to be knowledgeable in musculoskeletal oncologic principles. He or she should be the surgeon that will perform further surgical procedures if necessary. Other members of this team include a musculoskeletal diagnostic radiologist, medical oncologist, musculoskeletal pathologist, plastic surgeon, clinical psychologist, and radiotherapist. One must use one’s best judgment in determining whether or not to refer the patient to a tumor specialist. Some cases clearly demand a referral, while others may not. To this end, when uncertain, it is possible to send the films to an orthopaedic oncologist for further review.
BIOPSY
The biopsy is the ultimate diagnostic technique to evaluate neoplasms of all kinds. All tumors must be treated with the utmost caution. Many methods exist, such as needle, incisional, and excisional biopsies, and each one has clear indications. Choosing the appropriate biopsy technique will prevent considerable obstacles both surgically and with patient recovery (10). Specifically, fineneedle aspiration (FNA), core biopsy, and open surgical biopsy (which may be performed in an incisional or excisional manner) constitute the mainstay of techniques. The needle biopsy may be taken with a FNA from the tumor or a core biopsy. Incisional biopsies remove small samples of tissue, whereas excisional biopsies remove the mass in its entirety. Lesions suspicious for malignancy are best evaluated using a needle or incisional technique, thereby limiting the possibility of normal tissue becoming seeded with neoplastic cells. The location of the initial biopsy should be in an area that will be removed if and when a future excisional biopsy takes place. When taking a bone biopsy, the overlying muscle, fascia, and other viable tissue should be well retracted, and any bone defect should be sealed with bone wax to achieve hemostasis. The frozen section should be sent to a pathologist trained and experienced in musculoskeletal oncology. For every surgical biopsy, whether incisional or excisional, a surgical pathology request form should be completed by the surgeon prior to the procedure and should include all pertinent clinical data to facilitate the most accurate diagnosis.
FINE-NEEDLE ASPIRATION AND CORE NEEDLE
The FNA biopsy can be performed on a lump or mass to determine its identity. The FNA is also used to monitor the affects of treatment on a known mass. The major benefits of performing these biopsies are safety, decreased expense, and reduced trauma when compared with open biopsies. If the aspirate is a quality sample, there is a good chance that a diagnosis can be made without more invasive techniques.
As with all surgical procedures, standard sterile technique precedes this procedure. Once the site is prepared, local anesthetic may be administered. It is not uncommon that local anesthesia is not used due to the size of the needle employed to obtain the sample. Most commonly used to obtain a sample using FNA is a 22-gauge needle on a standard sterile syringe. Aspiration may be performed in one of two ways. One method is performed under fluoroscopy and utilizes two separate needles for better localization of the tumor. The cells are then aspirated by manually drawing back on the syringe of the needle that appears to be the most properly placed. The second method involves a rapid pass of one needle within the same track while a firm vacuum is maintained to prevent release of any potentially dangerous cells within the body. No matter the method, the specimen is sent immediately to pathology for smear. The cytopathologist will air-dry the smear for Giemsa staining and fix it in 90% ethanol for hematoxylin and eosin staining (11).
Guidance from other imaging modalities is frequently employed when performing FNA biopsies. Ultrasound-guided FNA may be attempted as an in-office procedure, but it must be performed by a trained operator, as well as a trained surgeon. However, it is not generally recommended that this be performed in the office because an experienced cytopathologist must be present. The cytopathologist introduces a small caliber needle into the lesion multiple times under vacuum, obtaining individual cells for analysis to hopefully provide immediate diagnosis. In addition to ultrasound, CT, MRI, and fluoroscopy are the most common. Fluoroscopy is advantageous because the images are dynamic.
There is controversy over the ultimate role FNA will have in musculoskeletal tumors, as it recovers individual cells and not tissue samples. Sample adequacy is always a concern. The small number of cells obtained may limit analysis to determine the biologic intent of those cells, being either benign or malignant. Immunohistochemistry analysis will often be able to help further classify cells into the parent lineage (fibrous, neural, lipoid, etc.) but will be less helpful in determining whether the tumor is benign or malignant.
Overall, the FNA procedure does not come without risks. Minor effects, such as soreness and bruising, may occur. Rarely, more serious complications, such as prolonged bleeding and infection can occur. Falsely negative results may result secondary to poor sampling. The most critical incident to avoid is seeding metastatic cells along the needle biopsy tract. This is very rare, with an incidence of 0.003% to 0.07% (12,13).
There are very few differences between the FNA and core needle biopsies (CNB). Patient preparation is the same for CNB as previously described for FNA. The major difference is the needle. Core biopsy needles are firm enough to penetrate the cortex of bone. A cylinder of bone, typically one-sixteenth of an inch in diameter and one-half-inch long, is extracted from the tumor. Because of this larger sampling, experts believe that this is a much more accurate means of diagnosing a tumor.
The choice of the actual core biopsy needle is determined after the internal consistency of the tumor is assessed with FNA. If the lesion is “hard” or has a denser texture, then a larger, stronger needle is chosen. There are a variety of needles to choose from, depending on the location of the lesion within the bone, integrity of the overlying bone cortex, and internal consistency of the target lesion (14). For “hard” lesions, a 15-gauge stiff bone-cutting needle is used. Roberts et al (15) found the RADI Bonopty coaxial drill and biopsy needle system (RADI Medical Systems, Uppsala, Sweden), with a 14-gauge sheath and eccentrically cutting 15-gauge drill tip, to be the most effective in penetrating intact cortical bone. This system appears to be a good choice when the lesion is intramedullary and covered by intact cortical bone. These procedures are most often performed by musculoskeletal radiologists under fluoroscopy or CT imaging.
Liu et al (14) recommends that FNA of hard lesions be performed, after core biopsy has been completed, with a needle 22-gauge or smaller. Since “soft” lesions usually yield little diagnostic tissue when sampled with bone-cutting needles, the vacuum syringe system may be combined with a bone-cutting needle, or substituted with a soft tissue biopsy needle to provide the highest biopsy yield for these soft lesions (16). A coaxial system should be considered when the lesion is deeper. This system will allow multiple passes through a stationary sheath. These multiple samples can help prevent nondiagnostic results arising from sampling a region of tumor necrosis or cystic degeneration.
It is optional to use a combination of FNA and CNB to obtain a definitive diagnosis almost 100% of the time (17). Ogilvie et al (18) found that in musculoskeletal tumors, core needle biopsy and FNA were clinically useful 66.7% and 68% of the time, respectively. However, combining these tests resulted in guiding definitive treatment as often as 80.6% (18). Domanski et al (17) identified 77 out of 78 malignant lesions using these two techniques together. The technique used to perform this procedure involves using a cannula and guide through a 2-mm skin incision with fluoroscopy to guide placement. The needle is then placed into the lesion after traveling through the cannula and suction is applied to extract a sample of tumor tissue.
There are many hazards associated with all biopsy techniques, especially with core biopsy of bone. Targeting of the lesion requires adequate imaging. For bone that is weakened by an underlying pathologic process, penetrating the cortex may be relatively easy. For more normal bone, penetrating the cortex may be difficult. This is also more difficult for the patient if done under local anesthesia. Frozen section is not possible with tissue samples containing significant amounts of bone, making adequate assessment of the sample at the time of the procedure difficult. Crush artifact, which may render the sample inadequate, may be created by the core biopsy as it cores through the involved bone. If there is a significant vascular component to the tumor, there is the possibility of significant bleeding and contamination of soft tissue with tumor cells, as the defect in the cortex cannot be contained. Any defect in the bone will weaken it. While a core biopsy hole is small, it may make up a significant portion of the circumference of a small bone of the foot and can therefore lead to pathologic fracture.
Finally, FNA can be of great use in those lesions in which there is a question of metastatic origin, such as lung or renal cell carcinoma. Both lung and renal cell malignancies develop distal, or acral, metastatic disease. Core biopsy techniques are more useful in accessible anatomic locations and in soft tissues. This technique is more difficult in the foot and ankle regions because of the limitations of soft tissue and the proximity of vital neurovascular structures.
OPEN BIOPSY
The use of a biopsy is needed to determine the proper diagnosis, as well as the stage of the tumor. In instances where FNA or CNB are not sufficient, an open incisional or excisional biopsy must be considered. Both incisional and excisional biopsies have similar surgical approaches but different indications and amounts of resected tissue.
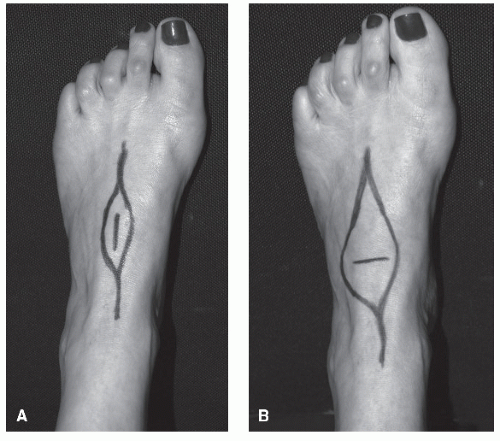
In undertaking an incisional biopsy, the surgeon needs to understand the anticipated surgical resection approach as to not compromise further surgery. Figure 94.1 shows the difference in soft tissue sacrifice due to a transverse biopsy versus the more appropriate longitudinal biopsy. Adequacy of the tissue is ensured by a frozen section analysis from an experienced pathologist. Limitation of the size of the incision and the hemostasis of the surgical site are important in reducing the complications of the biopsy. One of the authors (Hudson Berrey) has had, on several occasions, to perform transtibial amputations due to resultant surgical site complications from improper biopsy technique. Many of these could have been avoided if a proper biopsy had taken place.
As mentioned earlier, incisional biopsies only take a portion of the lesion. A core of bone is removed from the tumor with a trephine and sent to pathology for identification. The most common reasons for this technique are that the less-invasive biopsy techniques do not yield a diagnosis, and the tumor’s size or
location prevents complete excision. Unlike FNA and CNB, the risk of tissue contamination is reduced with incisional biopsies.
location prevents complete excision. Unlike FNA and CNB, the risk of tissue contamination is reduced with incisional biopsies.
When tumors can be identified as benign, excisional biopsy is the procedure of choice. This technique involves removing the tumor in total, or en bloc. Adequate visualization of the tumor intraoperatively is essential to performing a proper resection. Location of the tumor dictates the type of anesthesia required. For example, enchondromas of the digits may be resected under monitored anesthesia care with local anesthetic. When the lesion’s presentation is more proximal in the foot or ankle, as may occur with an interosseous lipoma of the calcaneus, general anesthesia is acceptable.
Another instance where open excisional biopsy is warranted is if the lesion is of limited size and located in an area where adequate margins may be obtained. If the lesion does not meet these conditions, a small incisional biopsy is preferred and should be placed in such a way that the entire biopsy track may be excised at subsequent surgery.
With incisional or excisional biopsy, the specimen should be tagged in such a way to identify the anatomic orientation of the tumor. These tags are usually small 2-0 nylon suture tails of varying length and number. This is carried out on the side table in the operating room and recorded on a specimen sheet by the circulating nurse. By giving as much information as possible about the specimen and its orientation in the body, the pathologist is able to better determine the margin’s adequacy and, hopefully, the benign or malignant nature of the tumor. Histologic diagnosis is often difficult in a frozen section due to tissue distortion during freezing and preparation of the slide. An important step in the course of the biopsy is to also culture the material obtained since there may be a significant amount of overlap between the clinical presentations of tumors and infections. Some advocate culturing every biopsy and sending a specimen for pathologic analysis when taking a culture for an infection since some tumors mimic the presentation of infection with pain, fever, erythema, and edema. Therefore, “culture every biopsy and get pathology on every infection.”
Once the tumor is visualized and compared with imaging studies, the surgeon should determine if the tumor penetrates the cortex. If there is no cortical infiltration, a window should be created to remove the tumor. Once the cortical window has been created, the lesion can be removed with either excision or curettage, which will be discussed later. The window should be made in a circular or oval shape, running longitudinally, with smooth contoured edges. Square or rectangular windows have a much higher frequency of stress risers (10). Clark et al (10) used cadavers to perform a study in femurs that showed that bone strength was greatly affected by the shape or size of the hole. Of the shapes studied, an oblong hole with rounded ends afforded the greatest residual strength (10). Furthermore, increasing the width of the hole caused a significant reduction in strength, while increasing the length did not (10). One should attempt to keep defects less than 10% of the bone diameter to maintain over 80% of bone strength (10). When biopsy size is greater than 20% of bone diameter, torsional strength decreases to 50% (10).
The deficit created by this resection should be made larger with a power burr so margins extend to healthy bone. Regarding resection margins, optimal surgical margins should allow 6 cm of healthy bone surrounding and 2 cm of healthy soft tissue. If a malignant tumor is responsive to adjunctive therapies such as chemotherapy, smaller resection margins may be acceptable.
Once the lesion is removed, concern moves to preventing recurrence and supporting the cortical shell. Some of the treatment options for prevention of recurrence include cryosurgery, (19) radiofrequency ablation (20,21 and 22), polymethylmethacrylate (PMMA) cementing, and phenol-alcohol cauterization (23). To provide stability to the structure, options range from PMMA to allograft or autograft. Demineralized bone matrix (DBM) has been mixed with the different grafts to help facilitate healing (24). Anderson and Ramsey (25) used the osteochondral autograft transfer system to fill the void created by resection of a chondroblastoma. A different challenge is presented when the tumor is in close proximity to a joint. The surgeon has to take this into consideration and create a cortical strut graft using a tricortical auto or allograft, corticocancellous chips, putty, gels, or injectable grafting material.
Hemostasis is essential prior to closure of the biopsy site to prevent seeding of neoplastic cells. Bone wax and electrocautery may be used to achieve hemostasis from bleeding bone. Placement of a drain can have grave consequences due to the possible spread of tumor cells, leaking them through more distal or proximal holes in other compartments prior to exiting the body. If drain placement cannot be avoided, it should be placed at the most distal or proximal end of the incision, not running the length of the incision. Robertson et al (26) evaluated the spread of microspheres following biopsies of canine bones to determine if passive dissemination of tumor cells exists. This study indicated that when the cortical window was left open, microspheres only spread to the adjacent tissue and the regional lymph nodes. Bone windows plugged with methylmethacrylate revealed little or no local spread of the microspheres in the adjacent tissue and regional lymph nodes. Significant microsphere activity was present in the lung within 20 minutes after placement of the methylmethacrylate plug (26).
Anderson and Ramsey (25) suggest that after resection of the tumor, instruments and gloves should be changed out in an additional precautionary measure to prevent seeding. Furthermore, copious amounts of irrigation followed by layered closure with clean instruments is performed to help reduce the risk of recurrence. The periosteum should be reapproximated whenever possible, as a part of this meticulous layered closure.
There are guidelines for bone tumor biopsy of the leg bones. For biopsies of the tibia, the patient should be supine with his or her leg rotated in a fashion that will expose the biopsy site upward. An anteromedial longitudinal incision should be made directly over the anteromedial cortex through the subcutaneous tissue (14). Care should be taken to avoid any vital structures in the area. The same care should be taken with the fibula, with the exception of the incision site. The incision should be lateral and longitudinal, just anterior to the posterior intermuscular septum (14).
Biopsies, regardless of type, are not without possible complications. A biopsy performed improperly may cause more harm to the patient, leading to potentially fatal outcomes. Mankin et al (27,28) found that approximately 19% of patients with primary musculoskeletal tumors required alteration of the optimum treatment plan as a result of biopsy-related issues. Although a suboptimal biopsy approach was only one of the many biopsyrelated complications, this statistic serves as an important reminder of the harm that may be the unintended consequence of an inappropriate biopsy. Furthermore, perhaps Mankin’s (28) most remarkable finding was that poorly planned biopsies led to unnecessary amputations in 5% to 8% of patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree