CHAPTER 86 Surgical Decompression for Herniated Nucleus Pulposus
INTRODUCTION
Lumbar herniated nucleus pulposus (HNP) falls within the spectrum of degenerative spinal conditions, and can occur with little or no trauma. Lumbar disc abnormalities increase with age.1,2 The actual incidence of lumbar disc herniations is unknown, as many people with herniations are asymptomatic.1,3,4 Ninety percent of lumbar herniations occur at the L4–5 and L5–S1 levels.5,6 More than 200 000 discectomies are performed in the United States each year.7 The success of this procedure, as with all surgical procedures, depends vastly on proper patient selection and to a lesser extent on surgical technique. However, it is incumbent on the spinal surgeon to be absolutely meticulous with intraoperative technique once the decision for surgery is made. To this end, The authors recommend the use of a microscope for lumbar discectomy. The authors believe that once the learning curve has been traveled, the microscope not only offers advantages over loupes, it forces one to think at a much higher level of clarity about what and where root encroachment pathology is present.8 More importantly, the patient has less morbidity and an earlier hospital discharge compared to standard or limited discectomy.6,9–14
PATHOPHYSIOLOGY
Intervertebral discs cushion and tether the vertebrae, providing both flexibility and stability. The normally gelatinous nucleus pulposus is surrounded by the ligamentous anulus fibrosus. In the young and healthy disc, the nucleus and anulus blend. Degenerative or pathologic changes can cause separation of the two entities, as well as compromise the integrity of the anulus, such that a sufficient load can cause nuclear fragments to migrate and impinge on neural elements.15 Lumbar disc herniations may occur with little or no trauma, although patients frequently report a bending or twisting motion as the inciting event, causing the onset of symptoms. Common causes of lumbar herniations include falls, car accidents, repetitive heavy lifting, and sports injuries of all types.
DIAGNOSIS
Imaging and other tests
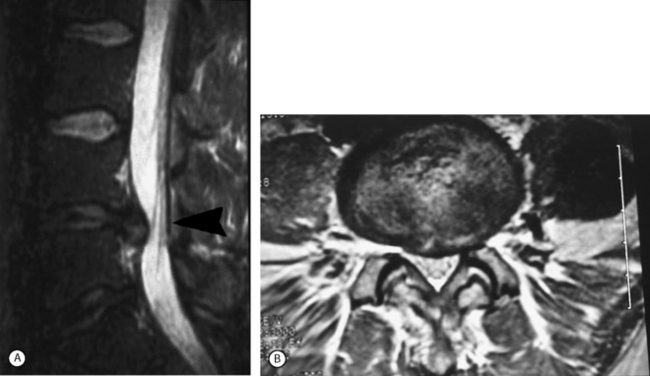
Magnetic resonance imaging (MRI) is the imaging study of choice to diagnose a lumbar disc herniation (Fig. 86.1). Plain radiographs should always be obtained. Patients who cannot obtain an MRI can be diagnosed using computed tomography (CT), CT myelogram, or CT discogram. These imaging tests are so sensitive that discectomy is not indicated if a disc is not found to be herniated by one of these techniques. Other tests can include an electromyogram (EMG) or nerve conduction study (NCS).
MANAGEMENT
It is important to understand that most patients with symptomatic herniated lumbar discs will get better over time regardless of the type of treatment. Weber’s classic study16 reported that sciatica from herniated nucleus pulposus (HNP) would improve 60% of the time with nonsurgical methods, and 92% of the time with surgery at 1 year. By 4 years out, he reported no statistical difference between the two groups, and no difference at 10-year follow up. In the absence of cauda equina syndrome, progressive or significant neurologic deficits, most practitioners attempt at least 4–8 weeks of conservative care before suggesting surgical intervention.
Indications for surgery
Surgical indications, as currently recommended by the North American Spine Society (NASS) include a definite diagnosis of ruptured lumbar intervertebral disc and:20,21
Conservative treatment consists of medical rehabilitation and interventional spine management and careful observation for at least 4–8 weeks. Some may benefit from a short trial of conservative treatment even after 8 weeks if no prior care was given. Failed conservative treatment is the most common indication for lumbar discectomy. Those who have not improved sufficiently and are not experiencing continued improvement might then be offered treatment by surgical excision of the disc. Such patients should be advised that this is an elective operation but that delay for longer than 3–6 months in the face of persistent and severe symptoms may ultimately compromise the best result.20,22
The latter four indications are exceptions to the 4–8 week rule. Excruciating pain may not be relieved by medical rehabilitation and interventional spine techniques and may require earlier surgical decompression. Recurrent sciatica should also receive consideration for surgery: the chance of recurrent sciatica after the second episode is 50%, and after the third episode is almost 100%.22 An example of a significant neurologic deficit may be a foot drop, or weakness that prevents normal posture, gait, or one that affects the patient’s profession or a particular skill. Any definite progression of neurologic deficit is an absolute indication for surgery. Cauda equina syndrome is relatively rare, being reported in 1–3% of patients with confirmed disc herniations.23,24 This is an orthopedic or neurosurgical emergency. Features include rapid progression of neurologic signs and symptoms, bilateral leg pain, caudal sensory deficit, bladder overflow incontinence or retention, and loss of rectal sphincter tone with or without fecal incontinence.
Contraindications for discectomy
The NASS and the American Academy of Orthopedic Surgeons (AAOS) have identified the following factors as absolute or relative contraindications for discectomy:20,25
SURGICAL PROCEDURES
One only has to review the natural history of lumbar disc disease to realize that spinal surgeons play a palliative role in the management of HNP.16,26–29 Surgical procedures as treatment for lumbar HNP include the following:
The use of an operating microscope
The attempt to improve visualization and illumination has led many spine surgeons to use loupes and a headlight. The authors believe the magnification and illumination built into the microscope offer many surgical advantages, the most important of which is reduced wound size and decreased tissue manipulation. The surgeon can limit the amount of tissue dissection by working through a small exposure directly over the pathology to be removed. Microsurgical techniques can also be used to preserve the ligamentum flavum and epidural fat to minimize postoperative epidural fibrosis and improve clinical results by preserving natural tissue planes.8,30 With this approach, the disc herniation can be easily removed, lateral recess stenosis can be decompressed, and nerve root manipulation is kept to a minimum. The senior author has used this technique since 1986 for most lumbar disc herniations, and has found the approach to be safe, with fewer dural tears and nerve root injuries and less postoperative epidural fibrosis than with standard discectomy.6 Table 86.1 lists the many advantages of the microscope over loupes.8,14,31
Table 86.1 Advantages of the Microscope Over Loupes
| Loupes | Microscope | |
|---|---|---|
| Magnification | Limited in amount, and fixed | Relatively unlimited and changeable during a case |
| Motion | Long surgery causes neck fatigue and motion of loupes | No motion of microscope |
| Focus | Each time surgeon looks up, refocusing is necessary to restart surgery | Microscope is in constant focus regardless of surgeon’s attention |
| Illumination | Not parallel to line of vision (paraxial) | Parallel to line of vision (coaxial), and stronger |
| Deep 3D vision | Limited when the skin incision is less than 65 mm (or surgeon’s interocular distance) | Maintained with even a 25 mm skin incision |
| Patient size | The larger the patient, the bigger the wound required | Neutralized (every patient is made the same size by the optics) |
| Teaching | Assistants excluded from vision | Assistants included |
| Surgeon’s neck | Fixed in flexion and requiring repositioning – fatigue during long surgeries | Spared – can be adjusted through inclinable binoculars |
The microscope is not without its disadvantages. Peripheral vision is lost, with the field of vision limited to approximately 4–5 cm. Because of this, the surgeon needs to know detailed anatomy of the spine. This is probably the biggest disadvantage of the microscope, although it in fact forces an increased awareness by the surgeon. The line of vision is fixed through the microscope. To look over structures (to overcome tissue overhang), the patient or microscope has to be adjusted during the surgery. This can be avoided by proper retraction or dissection of tissue away from the line of vision. Focusing of the microscope has to be done manually, unlike the surgeon’s own eyes. The shortcut to maintaining focus under the microscope is to have the anesthesiologist pump the table up or down as needed. Large instruments can block the line of vision, and the surgeon may need to look from outside the microscope periodically if this happens. Wilson et al. reported increased disc-space infection after microsurgery.32,33 This is most likely due to contamination from unsterile parts of the microscope during surgery although, as McCulloch and Young astutely point out, no one has looked at the potential for an increased infection rate when two surgeons with loupes and headlights bump heads over the wound! Recent reports by those who have long experiences with the microscope do not show any increased infection rates.6,12,14,34
Lumbar microdiscectomy
Microscopic discectomy (microdiscectomy) has become the gold standard for operative treatment of lumbar disc herniations, and the latest minimally invasive percutaneous techniques have not been shown to be more effective.8,35,36 Although no statistical differences can be shown in the ultimate long-term outcomes of microscopic versus standard open discectomies,10–12,29,37–39 the microscope provides improved illumination and magnification, and patients have less morbidity and earlier hospital discharge when compared to standard discectomies.6,9–14
1. Operative setup
General anesthesia is preferable because of patient comfort, airway, and sedation control. Another advantage is the option of hypotensive anesthesia. The procedure can also be done under epidural or local anesthesia with sedation, although this is not the authors’ preference. The patient’s position is always prone with the abdomen free, thus relieving pressure on the abdominal venous system and, in turn, decreasing venous backflow through Batson’s venous plexus into the spinal canal. This has the effect of decreasing bleeding from the epidural veins intraoperatively. Several frames are available for this, but the authors prefer a Wilson frame on a regular operating table because of the ease of setup. The frame is cranked up to induce flexion and opening of the interlaminar space. It is important to place the approximate spinal level of interest at the apex of the Wilson frame so the interlaminar space flexes open. When cranking the frame for increased flexion, careful attention must be paid to the position of the patient’s head and neck, as the body of the patient tends to be lifted up, thus increasing neck flexion. Additional padding may be necessary to stabilize the head and neck in a neutral position. Padding underneath the shoulders may also be needed to prevent shoulder subluxation or dislocation.
3. Skin incision and interlaminar space exposure
A 2–3 cm incision is made midline or up to 1 cm lateral to the spinous process on the symptomatic side, at a level directly over the disc space based on the localizing lateral radiograph. At L5–S1 this incision tends to be directly over the interlaminar space, but as one moves up the lumbar spine, this incision will be progressively over the cephalad lamina. The dissection is carried down to the lumbodorsal fascia, which is sharply incised. The fascial incision is placed carefully just lateral to the spinous processes to avoid damage to the supraspinous–interspinous ligament complex (Fig. 86.3) and to make it easier for lateral retraction. The subperiosteal muscle dissection and elevation are confined to the interlaminar space and approximately half of the cephalad and caudad lamina. The facet capsules are carefully preserved. A Cobb elevator and bovie cautery are used. It is important to watch out for spina bifida occulta while using the Cobb for subperiosteal dissection, especially at the L5–S1 level. A framed retractor is then placed. The medial hook is usually one size smaller than the lateral muscle blade to prevent tilting of the retractor frame. Expose the lateral border of the pars as a landmark for preserving enough of the pars during laminotomy to prevent fracture.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree