14 Resection of primary spinal or paraspinal tumors with direct spine invasion typically requires complex and at times innovative reconstruction techniques. Furthermore, for primary tumors for which curative intent is typically the main objective, long-term durability is paramount to reconstruction goals. Reconstruction of single or multiple vertebral body en-bloc resections involving more than one third to one half of the vertebra (e) and unilateral or bilateral posterior column resection typically involve extensive instrumentation and structural grafts or grafts to fill the osseous spinal defect(s). Specific to structural graft selection, the goal is to provide durable structural support across the defect(s) and achieve biological integration with the host bone (i.e., fusion). This chapter discusses factors that should be considered for structural graft selection and the options available for reconstructing spinal defects following primary tumor resection. Due to the rarity of primary spinal tumors, most relatively large case series describing reconstruction and graft selection techniques are surgeon/center specific, using a variety of graft selection and surgical techniques that are often unique to each patient and anatomic defect requiring reconstruction.1–3 The majority of reports understandably focus on the oncological outcome and technical aspects of each case; however, very few provide any long-term outcome information specific to the graft selection or reconstruction durability.1,2 In addition, due to clinical and technological advancements, the reconstruction techniques and graft choices have evolved within different centers that perform a higher volume of these cases.1–3 Consequently, there is gross variability in graft selection and reconstruction techniques from surgeon to surgeon and from center to center. Also, there are numerous case reports describing innovative and very complex reconstruction for very large tumors that push the limits of resection and reconstruction expertise. Although crucial to the understanding of rare conditions or procedures, these case reports do not provide us with generalizable information regarding structural graft selection and are often limited by relatively short-term follow-up. To provide a contemporary and broad representation of decision making in these complex reconstructions, I and my colleagues C.P. DiPaola and C.G. Fisher recently conducted an international Web-based survey to elicit expert opinion regarding decision making about the type of bone grafting choice for reconstruction following resection of primary spinal tumors. Thirty-one (61%) of the 51 members of AO Spine North America–Spine Net Oncology and AO International Oncology Knowledge Forum responded. The respondents were equally divided between orthopedic surgeons and neurosurgeons. The majority of respondents (71%) have been in clinical practice for over 10 years. The average number of primary spine tumor cases performed per year by the respondents in this group was 16, with a range of 2 to 100. Comparatively, the average number of metastatic cases per year for this group was 35, with a range of 7 to 150. The response for each survey question varied from 28 to 31/31 respondents. For primary tumors, the majority (93%) of respondents attempt to generate a fusion, with 63% of them noting that they use bone graft or bone graft substitutes every time and 30% using them almost every time. The preferred choice of bone graft material was equally split between morselized iliac crest (39%) and allograft (39%). The use of local bone alone was the preferred choice of only 16% of respondents. Ceramics (e.g., calcium phosphates) were not preferred by any respondent, and off-label use of bone morphogenetic protein (BMP) was preferred by only two (6.5%) respondents. The majority of respondents typically used combinations of morselized iliac crest, allograft, and local bone. When anterior structural support was required, structural allograft was preferred by 47% of respondents, followed closely by a prefabricated prosthetic replacement device (i.e., a “cage”) preferred by 40%. The use of vascularized fibula or rib was preferred by only three (10%) respondents, and polymethylmethacrylate (PMMA) was preferred by no one (Table 14.1). In the event of a 360-degree spondylectomy, the respondents overwhelmingly reported routine anterior column and bilateral posterior column reconstruction, with 42% doing so every time and 42% doing so almost every time. Anterior column reconstruction only and primary spinal shortening (i.e., host bone-on-bone, for a single-level resection) were occasionally/sometimes performed by 30% and 19% of respondents, respectively. Table 14.1 Structural Graft Selection: Summary of International Survey
Structural Graft Selection

 Introduction
Introduction
 Survey of Practitioners
Survey of Practitioners
| Graft Choice* | Preferred by Respondents (%) | Author/Editor Consensus |
| Structural iliac crest | 3.3% | Will consider for 1- to 2-level cervical reconstruction, or 1-level thoracic, particularly with associated radiation. Donor morbidity must be discussed with the patient. |
| Structural allograft | 46.7% | Preferred if available. Provides excellent integration, enables accurate tumor surveillance and lower cost than prefabricated prosthesis, but has limited availability and is susceptible to fracture if unprotected by instrumentation. |
| Prefabricated prosthetic replacement (i.e., cage) | 40% | Preferred. Greater flexibility regarding sizes, shape (can also get custom or modular implants), and availability compared with allograft. Metal cages create imaging artifact, which may delay detection of local recurrence. |
| Bone cement | 0% | Will consider in patients with short life-expectancy (palliative/intralesional procedure) and/or very low likelihood of fusion (e.g., high-dose postoperative radiation will be required). |
| Vascularized fibula or rib | 10% | Will utilize when there is a high risk of nonunion, in a revision case, or to supplement or provide soft tissue reconstruction (i.e., myo-osseous or osteocutaneous flap). |
 General Factors to Consider in Graft Selection
General Factors to Consider in Graft Selection
As noted above, there is often significant caseto-case variability even from the same surgeon or center. This is due to a variety of key factors that need to be considered when deciding on an optimal graft selection. These factors can be broadly categorized into patient, local, tumor, and health system factors. Patient factors include, but are not limited to, age, medical comorbidity, and life expectancy. Local factors can include the anatomic location of the lesion (discussed below), the circumferential and longitudinal degree of the defect, the bone quality, the adequacy of soft tissue coverage, and preand postoperative radiation. In addition, when considering autologous grafts such as an iliac crest or a vascularized osseous or composite graft, the morbidity and functional consequences from the donor site also need to be considered and weighed against alternatives to the local reconstruction needs. Tumor factors include histology, perioperative chemotherapy, ability to achieve negative margins, and the need for lifetime local surveillance. Health system factors include, but are not limited to, available multiteam expertise (e.g., for microvascular flaps or resection and reconstruction of associated vascular or visceral involvement), graft availability, and cost.
In our survey we also asked the experts to rank, in order of importance, the factors that influence their decision regarding graft selection. The top five factors ranked as extremely important in graft selection were patient’s life expectancy, achieving a clear margin, tumor histology, bone quality, and adequacy of soft tissue coverage (Table 14.2). The top three factors ranked as moderately important were graft availability, grafts that enable local tumor surveillance, and pre- or postoperative radiation therapy. A detailed discussion of each factor is beyond the scope of this chapter, but the following discussion provides a brief perspective on the key issues for each of the top factors.
Table 14.2 Factors that Influence Bone Graft Selection: Summary of International Survey
| Decision Making Factors* | Percent of Respondents |
| Extremely important: | |
| Life expectancy | 63.3% |
| Likelihood of achieving a clear margin | 51.5% |
| Tumor histology | 43.3% |
| Bone quality | 43.3% |
| Adequacy of soft tissue coverage | 43.3% |
| Moderately important: | |
| Graft availability | 56.7% |
| Enable local tumor surveillance | 53.3% |
| Radiation therapy pre- or postoperative | 46.7% |
A short life expectancy, not achieving a clear margin, and tumor histology typically relate to a higher likelihood of persistent or recurrent tumor and the probable need for ongoing adjunctive treatment (e.g., postoperative radiation) or future surgery.4 In these scenarios, a more inert graft such as PMMA that will be resistant to biological effects of local treatment and tumor invasion should be considered. Poor bone quality may necessitate consideration of techniques that utilize PMMA to augment or incorporate adjacent segments into the anterior structural graft and increase the required number of posterior fixation levels.3 Adequate soft tissue coverage is required to maintain or enable local vascularization and healing as well as reduce the likelihood of wound breakdown and infection. In this scenario a vascular graft would be given high consideration if adequate coverage were not achievable by other means.2 Graft availability and need for adequate tumor surveillance are self-evident. The negative effects of radiation on soft tissue healing are well known. However, preoperative radiation alone may not significantly adversely affect local bony healing, but it certainly results in local osteopenia.5 Acute postoperative radiation (planned or due to intraoperative tumor contamination or unexpected positive margin) does have a profound effect on bony healing of anterior interbody strut grafts.5 In the scenario where the need for acute postoperative radiation is likely or is imposed due to positive margins, the use a vascular graft or PMMA, depending on other factors previously discussed, should be considered.
 Specific Considerations Based On Anatomic Site
Specific Considerations Based On Anatomic Site
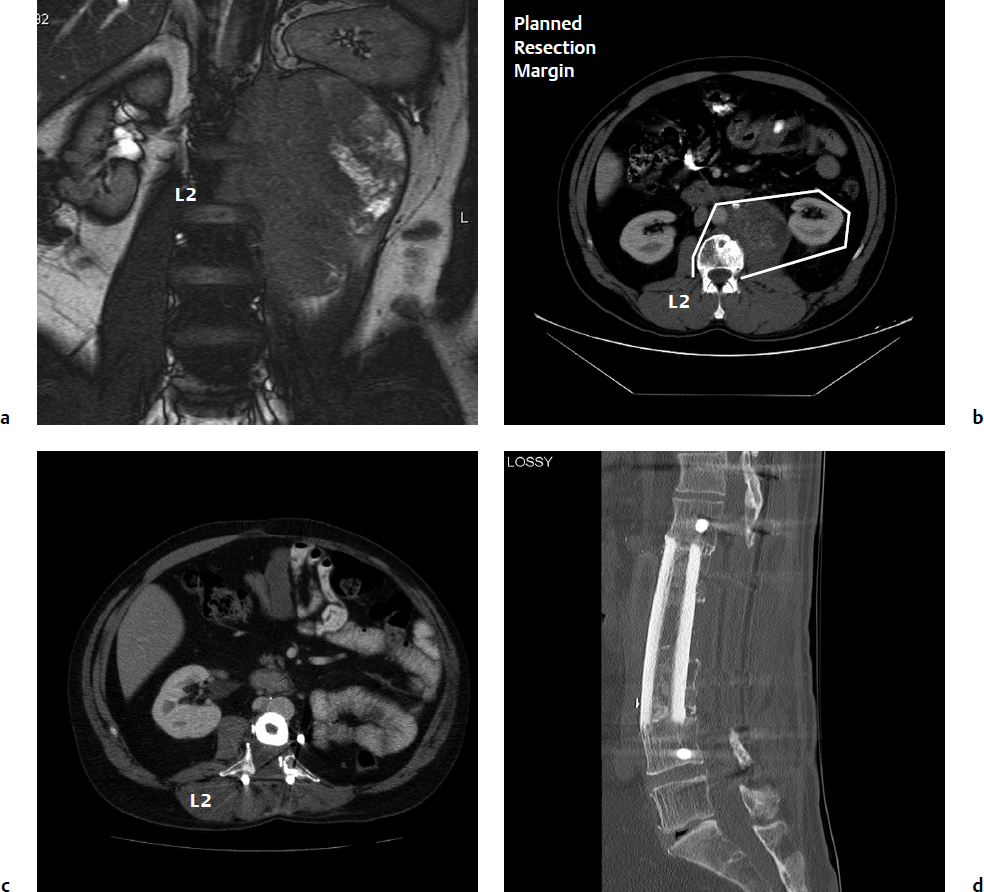
As noted above, the recommended structural graft choices (Table 14.1) in most regions of the spine are typically allograft or prefabricated cages (packed with allo- or autograft bone) that are appropriately sized with respect to diameter (preferably contacting the apophyseal rim of the endplate) and length. Structural bone allograft has the advantage of direct biological fusion (Fig. 14.1) where graft union with the host is possible.6 Allograft also enables unimpeded imaging for assessment of fusion and tumor surveillance. For structural allografts, the options are as follow: for the cervical spine—radius, fibular, or ulna; for the thoracic spine—tibia or humerus (upper thoracic); and for the lumbar spine—tibia or femur.
Perhaps the greatest current limitation of structural allograft pertains to its relative availability. Due to the possible risk of disease transmission as a result of poor regional procurement and sterilization processes or cultural beliefs, allograft is not available in many countries. Specific to the reconstruction of segmental defects, there is an associated risk of fracture, particularly for irradiated or freezedried allograft.6 However, due to the load sharing and torsional stability that is offered by spinal instrumentation, this is an uncommon occurrence in spinal cases. Comparatively, prefabricated prostheses often referred to as “cages” offer the advantage of greater availability and more size and shape options. Furthermore, for complex defects with transitional anatomy such as at the cervicothoracic junction, contoured or custom options are available.
Most prefabricated prostheses do not directly integrate with the host, and consequently need to be packed with morselized bone (auto- or allograft) to enable fusion. For primary tumor reconstruction, metallic cages have the distinct disadvantage of metal artifact on magnetic resonance imaging (MRI) and to a lesser extent on computed tomography (CT), which may delay the detection of local disease recurrence on tumor surveillance (the clinical sequelae of which can be debated). Furthermore, the cost of cages, particularly contoured or custom devices, may be prohibitive in certain health care systems. Reconstruction of complete unior bilateral posterior column defects is also variable from case to case, but appropriately sized load-sharing strut graft(s), typically alloor autograft, are preferable. At our center, we have had excellent success with using rib grafts for this purpose.7 In cases where there is a high risk of failure (revisions or postoperative radiation), a vascularized rib can be considered.
Junctional regions of the spine typically present with further unique anatomic and mechanical challenges that warrant discussion. Due to the frequent need for massive resections, reconstruction of lumbar-pelvic defects typically represents the most variable and difficult reconstructions, with the greatest risk of complication. This topic is discussed in Chapter 13. Due to the straight alignment, structural graft selection at the thoracolumbar junction does not present any additional specific issues beyond those already discussed. On the other hand, the transitional anatomy (rapid change in size and contour) of the cervicothoracic junction (CTJ) often requires the use of contoured cages or unique allograft step-cuts, particularly for multilevel resection that cross the CTJ. The occipitocervical junction (OCJ) is associated with unique access and reconstruction challenges for marginal or wide en-bloc resections.8 This is particularly so for resections that require high anterior access and the possible resection of the posterior pharyngeal wall, wherein adequate soft tissue coverage is critical to avoid infection and enable osseous healing. In the latter scenario, a myo-osseous vascularized fibular strut graft (i.e., with attached muscle and fascia) is recommended to provide structural support (clivus to cervical spine) and posterior pharyngeal wall reconstruction (Fig. 14.2). Alternatively, if the posterior pharyngeal wall is intact, then posterior reconstruction with cages or autograph struts from the occipital condyles to the cervical lateral masses can be utilized due to the sagittal vertebral axis being posterior in the upper cervical spine.9 The terminal ends of the cages can be compressed into the condyle and cervical lateral mass to provide immediate stability of the graft.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree