Michelle Stern
Antigone Arygriou
Roshni Durgam
Jennifer Gomez
Hejab Imteyaz
Rakhi Sutaria
![]()
13: Stroke
![]()
PATIENT CARE
GOALS
Provide patient care that is compassionate, appropriate, and effective for the treatment of a patient with stroke and the promotion of health.
OBJECTIVES
1. Describe the key components of the assessment of the patient with stroke.
2. Discuss the long-term outcomes of stroke.
3. Assess the impairments, activity limitations, and participation restrictions associated with stroke.
4. Describe the psychosocial, vocational, and educational aspects of stroke.
5. Describe potential injuries associated with stroke.
6. Formulate the key components of a rehabilitation treatment plan for the patient with stroke.
Issues that are relevant to patient care involve understanding the medical management of these patients in the acute care setting and beyond. But it begins with a thorough history and physical examination for these patients.
When taking the medical history of someone with a stroke, a chronological description of the development of the condition should be included. Risk factors associated with the stroke (both nonmodifiable and modifiable risk factors) should be assessed. Any prior workup to determine the cause (ischemic vs. hemorrhagic) should be identified.
In obtaining the rehabilitation history, it is important to ascertain the prior level of functioning, interests, avocations, vocation, social supports, and living environment. Review of systems should include all the systems as they may all be affected from both the acute and late effects of stroke.
Key elements of the physical examination particularly specific to stroke include assessments of the neurological and musculoskeletal systems. Neurological assessment should include motor and sensory testing, an evaluation of limb spasticity, swallowing, language issues, visual findings, and neglect. There are different syndromes determined by the location of the stroke. Determining the location and type of stroke will help guide the rehabilitation professional to determine the type of rehabilitation needed. Patients also need to be evaluated to determine the level of rehabilitation care they will need. Depending on their current and prior level of function, patients may require outpatient, acute, subacute, or long-term care following a stroke.
Assessment of rehabilitation needs and readiness for rehabilitation participation should, at a minimum, include the following:
Medical Stability
1. Medical workup and treatment plan
2. Stable vital signs for 24 hours
3. No chest pain within the past 24 hours, with the exception of stable angina or documented noncardiac conditions
4. No significant arrhythmia
5. No evidence of deep vein thrombosis (DVT)
Rehabilitation Needs
1. Cognitive capability of participating in rehabilitation
2. Willingness to participate in rehabilitation services
3. Adequate prior functional status
4. Capacity
It is important to determine whether the patient will be able to tolerate a 3-hour rehabilitation program and whether he or she has the ability to retain information. While under a physiatrist’s care, the patient’s decision-making capacity should be assessed. The key components of this are multifaceted—and it revolves around the patient’s understanding of his or her condition and treatment options. The patient should be able to ascertain how it affects him or her and be able to express choices made. In stroke patients, this may prove to be rather difficult, given possible cognitive deficits and/or aphasia. Ultimately, clinical judgment prevails.
The patient’s medical condition should be evaluated for level or complexity to determine if he or she will require a higher level of medical monitoring. The patient’s support systems including friends and family in the community should be identified to ensure that the patient will have the necessary support to enable him or her to return to the community safely. The likelihood of motor recovery in a timely fashion should also be determined. For medical monitoring the clinician needs to think about preexisting medical illnesses that necessitate ongoing care (e.g., hypertension, diabetes mellitus [DM]), secondary post-stroke complications (e.g., deep venous thrombosis, pneumonia), or acute poststroke exacerbations of preexisting chronic diseases (such as angina in a patient with ischemic heart disease). Management of these conditions can constitute major portions of the rehabilitation effort. Some patients may be more disabled by certain associated comorbid diseases than by the stroke itself. Some medical problems, such as heart disease, have been found to affect the course and outcome of rehabilitation adversely following a stroke. Medical complications can limit the patient’s ability to participate in therapeutic exercise programs, inhibit functional skill performance, and reduce the likelihood of achieving favorable outcomes from rehabilitation. The rehabilitation interventions might also affect the medical condition adversely, causing an exacerbation of the disease or necessitating an adjustment in the treatment program. Patients who are treated in a stroke unit have medical complications that frequently occur during the postacute phase of rehabilitation, affecting up to 60% of patients (and up to 94% of patients with severe lesions) (1). Common medical complications have varied in different reports, but the most common complications seen after a stroke include aspiration, pneumonia, urinary tract infection, depression, musculoskeletal pain, complex regional pain syndrome (CRPS), falls, malnutrition, venous thromboembolism, and pressure ulcer (1).
RETURN TO COMMUNITY
National Stroke Association guidelines estimated that 10% of stroke survivors recover almost completely, 25% recover with minor impairments, 40% experience moderate to severe impairments requiring special care, 10% require care in a nursing home or other long-term care facility, and 15% die shortly after the stroke (2). Deutsch et al. concluded that patients with stroke, mild motor disabilities, and mild to no cognitive disabilities; patients with moderate and significant motor disabilities; and older patients with severe disabilities were significantly more likely to return to community from an inpatient rehabilitation facility rather than from a skilled nursing facility (3).
Some patients after a stroke are able to return to work (RTW). Treger noted in his article “Return to Work in Stroke Patients” that the reported rate was 19% to 73% (4). Patients more likely to RTW are those aged less than 65, a high education level, and white-collar employment. A negative predictor was the severity of the stroke. The clinician needs to work with the patient and realistically look at his or her job duties to determine if the patient will be able to RTW. The physiatrist may have to work with employers for modified work schedules on RTW. Those who are of working age and who are unable to work may need to be referred for disability.
Many stroke survivors belong to the retirement age, but one-third are younger than 65 years. In stroke patients younger than 65 years who are in the workforce, returning to work is associated with a sense of independence both socially and financially, and sense of well-being. Thus, for these younger stroke patients, RTW and community is an integral part of their rehabilitation goals in stroke recovery (5). There are some factors that help in decision making to RTW such as working years until retirement, occupation, and financial status (6). Studies have shown that weakness, neurological deficit, spasticity, and cognitive and speech impairment are negative predictors of RTW (7). In addition, activities of daily living (ADLs) is a strong predictor of RTW. The estimated rate of RTW varies widely from 11% to 85% in stroke population (4,8). Thus, it is imperative to take into account the needs and limitations to RTW in stroke survivors and set rehabilitation goals to attain maximum functional independence. The assessment of the workplace to accommodate the stroke patient function is part of the goal assessment.
Another concern for the poststroke population is return to driving. Driving requires vision, cognition, and muscular strength and physical function (9). Studies have shown that in stroke patients, fatigue, strength, and motor activity play a critical role in resumption of driving. Stroke patients might need driving evaluations, training, and adaptation to vehicles in order to return to safe driving. Rehabilitation interventions should focus on achieving safe driving assessment and training.
STROKE-RELATED IMPAIRMENTS
The risk of death after stroke is greatest in the first month and less for ischemic stroke than for hemorrhagic stroke. Thirty-day mortality after ischemic stroke is approximately 20% (10).
Death is more likely to occur from medical complications than from neurological complications. Those surviving have an increased death rate than age-matched controls. Recurrent stroke is a major cause of long-term morbidity and mortality. Recurrence is highest in patients with atherosclerosis and lowest in those with lacunar infarcts. Majority of stroke recovery occurs in the first 3 to 6 months, but cognitive and language deficits may continue to improve for up to a year later. Within 3 to 6 months, more than 85% of stroke survivors walk independently, two-thirds are independent with ADLs, and more than one-third have minimal disability (11). Bard and Hirschberg asserted that if no initial motion is noticed during the first 3 weeks or if motion in one segment is not followed within a week by the appearance of motion in a second segment, the prognosis for recovery of full motion is not favorable (12).
Factors predicting poor ADL outcome include the following: advanced age, myocardial infarction, DM, severe weakness, poor sitting balance, visuospatial deficits, cognitive changes, incontinence, and low initial ADL scores. Approximately one-third of patients with acute stroke have clinical features of aphasia. At 6 months or more after stroke, only 12% to 18% of patients have identifiable aphasia (13). Skilbeck and colleagues reported that patients with aphasia continue to show some late improvement in language function even more than 1 year after onset (14).
Patients who are classified initially as having Broca aphasia have variable outcomes. In patients with large hemisphere lesions, Broca aphasia persists with little recovery. Patients with smaller lesions confined to the posterior frontal lobe often show early progressive improvement, but the impairment may evolve into a milder form of aphasia with anomia and difficulty in finding words. Patients with global aphasia tend to progress slowly, with comprehension often improving more than expressive ability does.
The communicative ability of patients who initially have global aphasia improves over a longer period of time, up to a year or more post onset. Patients with global aphasia associated with large lesions may show only minor recovery, but recovery may be quite good in patients with smaller lesions. The extent of language recovery associated with Wernicke aphasia is variable.
The Stroke Unit Trialists’ Collaboration Cochrane Review (updated in 2001) concluded, “Patients receiving organized inpatient stroke unit care were more likely to survive, regain independence, and return home than those receiving a less organized service” (15). The Cochrane review further concluded, “Acute stroke patients should be offered organized inpatient stroke unit care, typically provided by a coordinated multidisciplinary team operating within a discrete stroke ward that can offer a substantial period of rehabilitation, if required; there are no firm grounds for restricting access according to a patient’s age, gender, or stroke severity” (15).
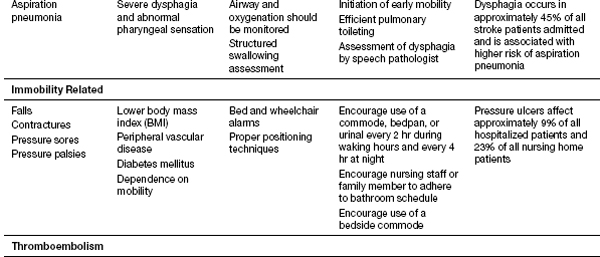
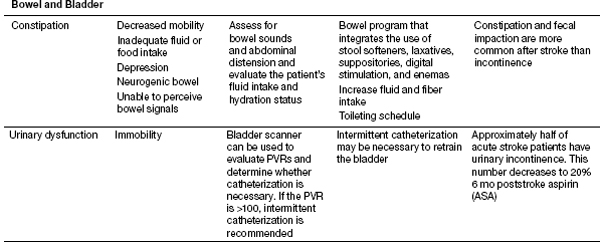
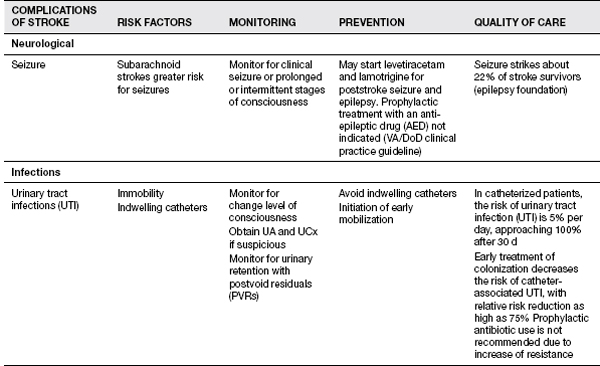
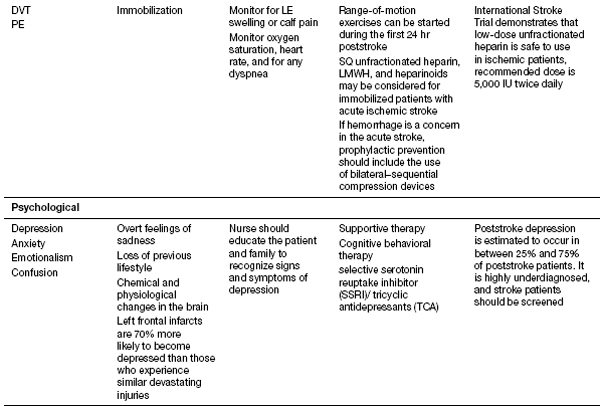
Recent studies from Thailand have helped shed better light on the medical complications during inpatient stroke rehabilitation as well as long-term morbidities in stroke survivors (16). Their experience showed that 70.3% of patients experienced at least one complication (16). Poststroke depression was found most commonly, followed by musculoskeletal pain, urinary tract infection, CRPS type 1, pneumonia, cardiovascular complications, falls, upper gastrointestinal bleeding, seizure, and pressure ulcers (16). History of myocardial infarction, low Barthel scores, urinary incontinence, indwelling catheters, and dysphagia were risk factors for complications. CRPS risk factor was limited shoulder range of motion (ROM). Their follow-up report looked at patients 1 year post stroke and evaluated the morbidities encountered. In order of the complications found 1 year post discharge were musculoskeletal pain, shoulder subluxation, depression, spasticity, joint contracture, urinary incontinence, dysphagia, pressure ulcer, infection, and neuropathic pain. Patients older than 60 years were more likely to develop complications. Please see Table 13.1 for the list of complications noted to occur with strokes; these should be kept in mind when treating stroke patients. Some of these topics will be discussed in further depth in this section.
TABLE 13.1 Complications of Stroke
Immobility
Immobility can lead to pressure ulcers, atelectasis, contractures, and DVT/pulmonary embolus (PE). To help reduce these risks, the patient should be mobilized as soon as medically appropriate. Patients should be evaluated for need for specialized beds and the nursing staff instructed on proper positioning of patients to reduce skin breakdown, limb edema, and contractures. Prevention of pressure ulcers depends on early identification of patients at risk and reliable implementation of prevention strategies for patients identified to be at risk. As per Berlowtiz, patients at highest risk for skin breakdown are those with (a) dependence in mobility, (b) altered sensation, (c) fecal and urinary incontinence, (d) excessively low or high body mass index, and (e) diseases associated with cachexia (17). A valid and reliable pressure ulcer risk assessment tool, such as the Braden Scale, can help predict the risk of pressure ulcer development and thus help the rehabilitation team implement interventions to prevent skin breakdown.
DVT prevention should include the use of low-molecular-weight heparin (LMWH), heparinoid, or unfractionated heparin (if not already on warfarin). Without prophylaxis, a DVT can develop in up to 50% of patients with severe stroke (18).
The PREVAIL study determined that LMWH (40 mg per day, starting between 24 and 48 hours after stroke onset) was superior to using 5,000 units of unfractionated heparin twice daily (19,20). In patients who are not ambulatory, antiembolic stocking (i.e., compression stockings) and intermittent pneumatic compression may enhance the benefit from heparin treatment to reduce the incidence of DVT and DVT complications. Prevention of PE with the use of retrievable or permanent inferior vena cava filters (IVCFs) may be considered for some patients who have contraindications to anticoagulation and are at high risk.
Dysphagia
All stroke patients should have an evaluation for their swallow function. Dysphagia along with impaired cough and gag reflex are risk factors for developing aspiration pneumonia (ASPNA). Dehydration and nutritional deficiencies can occur as well. A formal swallow evaluation with video fluoroscopy or FEEST (flexible endoscopic evaluation of swallowing with sensory testing) may be required. The clinician should also be aware of the modified textures of food and liquids that are available at their facility and order appropriately. A nasogastric tube may be needed in some patients temporarily, and severe dysphagia or cognitive impairments may necessitate a PEG (percutaneous endoscopic gastrostomy tube) placement. Patients with decreased level of consciousness, multiple infarcts, brainstem infarcts, or large infarcts are at greatest risk for aspiration. The clinician can do a water swallowing test at bedside, with coughing, choking, or a wet voice after drinking being suggestive of aspiration.
Falls
Reported rate of falls is 40% in patients (21). Depression, cognitive impairment, and sensory deficits are increased risk factors for falls. Most falls occur when trying to transfer out of a wheelchair. There is also a high risk of falls in this patient population in the community, with 14% of patients falling, with the highest incidence in the first 6 months post discharge (21). It is also important to minimize medications that may cause orthostatic hypotension (e.g., polypharmacy) as well as to maintain proper hydration.
Orthostatic Hypotension
Orthostatic hypotension is a potential adverse effect of drugs used currently in the treatment of cardiovascular conditions such as hypertension and cardiac arrhythmias. Caution must be taken in the use of these medications as many have a diuretic effect, which may induce orthostatic hypotension. However, orthostatic hypotension is more common with medications such as alpha-1 blockers, adrenergic blockers, and centrally acting drugs. Drugs used for the treatment of psychiatric illnesses are all associated with orthostatic hypotension. See Table 13.2—orthostatic medications. The table lists common medications associated with drug-induced orthostatic hypotension.
The medical conditions for which the aforementioned medications are used are very common. Appropriate screening and monitoring of orthostatic hypotension should be routinely carried out to prevent falls, injuries, and further adverse effects.
Urinary Incontinence
Forty to sixty percent of people admitted to the hospital after a stroke can have problems with urinary incontinence, with 25% of stroke survivors still having problems on hospital discharge, and 15% remaining incontinent after 1 year (22). Increased age, increased stroke severity, the presence of diabetes, prostate hypertrophy in men, preexisting impairment in urinary function, and the occurrence of other disabling diseases increase the risk of urinary incontinence in stroke. Stroke survivors usually develop a hyperreflexic bladder. The development of a urinary tract infection can occur with prolonged catheter use, alterations in bladder emptying, or reduced fluid intake.
TABLE 13.2 Orthostatic Medications
MEDICATION CLASS | MEDICATIONS |
Alpha-1 antagonists | Terazosin Prazosin Doxazosin |
Antihypertensives/vasodilators | Angiotensin-converting enzyme inhibitors Beta-blockers Alpha-beta-blockers Calcium channel blockers Clonidine Hydralazine Methyldopa Nitrates Dipyridamole Reserpine |
Diuretics | Hydrochlorothiazide Loop diuretics |
Anticholinergics | Oxybutynin Ditropan |
Phosphodiesterase type 5 inhibitors | Sildenafil Vardenafil |
Antidepressants | Tricyclic antidepressants Trazodone Monoamine oxidase inhibitors |
Muscle relaxants | Baclofen Tizanidine Cyclobenzaprine Methocarbamol |
Opioids | Morphine Oxycodone Tramadol |
Constipation
Constipation and fecal impaction are more common after stroke than fecal incontinence. Immobility and inactivity, inadequate fluid or food intake, depression or anxiety, a neurogenic bowel, constipating side effects of medications, the inability to perceive bowel signals, lack of transfer ability, and cognitive deficits may each contribute to this problem. Goals of management are to ensure adequate intake of fluid, bulk, and fiber and to help the patient establish a regular toileting schedule. Stool softeners and judicious use of laxatives may be helpful. Patients on tube feeds may develop diarrhea. Fecal incontinence that does not clear 2 weeks poststroke is a poor prognostic indicator (23).
Spasticity
Sixty-five percent of stroke survivors develop spasticity after a stroke (24). Treatment options include stretching, ROM, and serial casting. While oral medication for spasticity is not as effective for spasticity from cerebral causes versus those of spinal origin, medication that can be used include baclofen, tizanidine, dantrolene and benzodiazepines. For long-term patients with spasticity, there is benefit from phenol and botulinum toxin injection. For more severe cases, intrathecal baclofen pumps have been used in select cases.
Hemiplegic Shoulder Pain
Lindgren found shoulder pain to be a frequent complication after stroke and impairs potential recovery and function (25). Poduri reports pain can occur as early as 2 weeks poststroke, but typically occurs 2 to 3 months after stroke (26). Suspected factors contributing to the syndrome include subluxation, contractures, CRPS, rotator cuff injury, and spastic muscle imbalance of the glenohumeral joint.
Depression
Major depression has been reported by Robinson and Spalletta to occur in 21.7% and minor depression in 19.5% of patients following a stroke (27). It is associated with worse long-term functional outcome after stroke, and treatment with antidepressant agents such as selective serotonin reuptake inhibitors have been shown to be beneficial.
Aspiration
An observational study assessed the factors that help decide the postacute level of care for stroke patients with ASPNA (28). It concluded that patients with ASPNA and a National Institutes of Health Stroke Scale (NIHSS) value of 7.44 or greater showed the need for additional postacute care. Those patients with ASPNA and an NIHSS value of 10.93 or greater showed the need for a skilled nursing facility or subacute care. Patients older than 69 years with ASPNA had increased chances of placement in subacute care (28).
Cardiac Precautions
A useful set of cardiac precautions in patients undergoing rehabilitation was developed by Fletcher et al. (29). Activity should be terminated if any of the following develops:
 New-onset cardiopulmonary symptoms
New-onset cardiopulmonary symptoms
 Heart rate decreases more than 20% of baseline
Heart rate decreases more than 20% of baseline
 Heart rate increases more than 50% of baseline
Heart rate increases more than 50% of baseline
 Systolic blood pressure (BP) increases to 240 mmHg
Systolic blood pressure (BP) increases to 240 mmHg
 Systolic BP decreases to ≥30 mmHg from baseline or to less than 90 mmHg
Systolic BP decreases to ≥30 mmHg from baseline or to less than 90 mmHg
 Diastolic BP increases to 120 mmHg
Diastolic BP increases to 120 mmHg
STROKE RECOVERY AND ROLE OF REHABILITATION
For patients further out from stroke, Pang found there is strong evidence that aerobic exercise (40%–50% heart rate reserve (HRR) progressing to 60%–80%) conducted 20 to 40 minutes and 3 to 5 days per week is beneficial for enhancing aerobic fitness, walking speed, and walking endurance in people who have had mild to moderate stroke and are deemed to have low cardiovascular risk with exercise after proper screening assessments (grade A recommendation) (30). The effects of aerobic exercise on other health outcomes require further study.
Motor pattern of recovery from stroke was discussed in the classic articles by Twitchell, who detailed stages of recovery from a flaccid state to a progressive increase in tone (31). It also details the synergy patterns that develop in the upper and lower extremities during the recovery phase. In middle cerebral artery (MCA) strokes, proximal recovery occurs before distal, lower extremity recovers before upper extremity, and synergy patterns develop before isolated voluntary movement. In a study of 188 patients with stroke, Nijland et al. found that assessment of finger extension and shoulder abduction within 72 hours after stroke can help to predict upper limb recovery (32). If, by the second day following stroke, patients in whom upper limb motor function was affected were capable of some voluntary extension of the fingers and some abduction of the hemiplegic shoulder, there was a 0.98 probability that they would regain some dexterity by 6 months. Patients with no such voluntary movement on the second day had only a 0.25 probability of regaining dexterity by 6 months. Full recovery at 6 months was achieved in 60% of patients with some early finger extension (29).
There are various different therapy techniques that have been used during the rehabilitation process. The most commonly used models consist of (a) traditional therapy, (b) Bobath concept—neurodevelopmental training (see Table 13.3), (c) proprioceptive neuromuscular facilitation (PNF) (see Table 13.4), and (d) Brunnstrom (see Table 13.5). Traditional therapy includes ROM, strengthening, mobilization, and compensatory techniques (see Table 13.3). In his article about the effectiveness of the Bobath concept, Kollen describes it as the most popular treatment approach used, but limited data to support its superiority (33). Persons with motor deficiencies following stroke are unable to direct nervous impulses to muscles in the different combinations used by persons with an intact central nervous system (CNS). The goal is to suppress abnormal muscle patterns before normal patterns are introduced. Abnormal patterns are modified at proximal key points of control, such as the neck, spine, shoulder, and pelvis. PNF stimulates nerve/muscle/sensory receptors to evoke response through manual stimuli to increase ease of movement and promote function. Brunnstrom encourages synergy patterns that develop after stroke through the use of cutaneous/proprioceptive stimuli. Other therapy protocols that are currently being evaluated include the use of constraint therapy as well as the role of robotics (see Table 13.6)
TABLE 13.3 Neurofacilitation Technique
Bobath’s Technique: Aims to inhibit spasticity and synergies, using inhibitory postures and movements, and to facilitate normal autonomic responses that are involved in voluntary movement |
TABLE 13.4 Proprioceptive Technique
Proprioceptive Neuromuscular Facilitation Technique: Uses quick stretching and manual resistance of muscle activation of the limbs in functional directions, which are often spiral and diagonal in direction |
TABLE 13.5 Brunnstrom Stages
Stage 1 | Immediately following the stroke is flaccid paralysis |
Stage 2 | Recovery begins with developing spasticity: At this time, development of minimal movement is seen in synergies |
Stage 3 | Spasticity becomes more pronounced and patient gains voluntary control through synergy pattern: Voluntary movement is synergy dependent |
Stage 4 | Spasticity begins to decline: Some movements come out of synergy |
Stage 5 | Spasticity continues to decline: Movement is almost independent of synergy |
Stage 6 | Normal movement with normal speed |
TABLE 13.6 Constraint-Induced Therapy
Forces the individual to use the impaired limb by constraining the unaffected extremity |
MEDICAL KNOWLEDGE
GOALS
Demonstrate knowledge of established and evolving biomedical, clinical, epidemiological, and sociobehavioral sciences pertaining to stroke, as well as the application of this knowledge to guide holistic patient care.
OBJECTIVES
1. Describe the epidemiology, anatomy, physiology, and pathophysiology of stroke.
2. Identify the pertinent laboratory and imaging studies important in stroke.
3. Review the treatment and management of stroke.
Taking care of stroke patients is a challenge for the rehabilitation specialist. There is a large spectrum of symptoms and outcomes for the different stroke syndromes. This chapter will focus on issues the provider needs to understand in order to best care for patients after developing a stroke. To understand how to manage patients with stroke, the clinician must be aware of the pathophysiology of the type of stroke that person has, the likelihood of recovery, and the long-term implications for the patient. We will first start off describing the various stroke subtypes and provide insight into what the clinician should be aware of.
It is imperative that the rehabilitation specialist is aware of the stroke subtype his or her patient has, as this will lead to differences in recovery and complications. Stroke can be divided into ischemic or hemorrhagic lesions, with the ischemic lesions being the predominant subtype (see Table 13.7). Though there are many different causes of stroke, this is out of the scope of this chapter. The most common causes and those seen most often in the rehabilitation setting will be discussed in this chapter.
Stroke is one of the four leading causes of death and the number one cause of severe neurological disability in adults. In the United States, there are four million stroke survivors and more than 750,000 new strokes each year (34,35). Risk factors for stroke include both modifiable and nonmodifiable causes. which are listed in Table 13.8.
TABLE 13.7 Types of Stroke
Ischemic lesion | Hemorrhagic lesion |
Thrombotic | Intracerebral |
Embolic | Subarachnoid |
TABLE 13.8 Stroke Risk Factors
NONMODIFIABLE RISK FACTORS FOR STROKE | MODIFIABLE RISK FACTORS FOR STROKE |
Age (most important risk factor) Risk increases after age 55 and doubles after each decade after 55 | Heart disease (congestive heart failure, coronary artery disease, valvular heart disease) Atrial fibrillation (increases risk of embolic stroke by 5) |
Race (African Americans greater than Whites greater than Asians) | Diabetes (twofold increased risk) Hypercoagulable states |
Sex (male greater than female) | Hypertension Cigarette smoking Carotid stenosis |
Family history | Hyperlipidemia Alcohol and cocaine use |
ISCHEMIC STROKE
An ischemic stroke is caused by focal cerebral ischemia. There are several different mechanisms that can disrupt blood flow in the brain. The neurological deficits manifested after stroke will depend on the vascular distribution affected by the stroke. Eighty-five percent of strokes are ischemic in nature (36). Ischemic strokes can be further subdivided into thrombotic or embolic.
The Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification system for ischemic stroke is based on the underlying stroke mechanisms (37):
1. Large artery atherosclerosis: Intracranial, extracranial (carotid, aortic arch): Usually occurs during sleep and patient awakens unaware of deficits. Deficits may have an insidious progression over the course of 24 to 48 hours.
2. Cardioembolic: The source of embolic strokes is most often cardiac in nature as up to 75% of all cardiac emboli travel up to the brain, causing an ischemic stroke. Cardiac conditions that can cause stroke include atrial fibrillation, segmental wall akinesis, paradoxical embolus, patent foramen ovale, and congestive heart failure. Atrial fibrillation alone is associated with a four to five times increased risk of ischemic stroke. The rapid cardiac fibrillations induce the formation of thrombi that can embolize and enter the cerebral circulation. Vegetations on heart valves in endocarditis or prosthetic heart valves can also be a cause. Embolic strokes have a sudden onset and usually occur when patients are awake.
3. Small vessel: Lacunar infarction Lacunes are small infarcts that may be seen in the putamen, pons, thalamus, caudate, and internal capsule. They are caused by the occlusion of small 50 to 200 mm arteries of deep penetrating branches of larger blood vessels. Lacunar infarcts are highly associated with hypertension. Since lacunar infarcts affect smaller vessels, they usually do not involve higher cortical functions, which remain intact following stroke.
4. Other: Vessel dissection, venous thrombosis, drug induced
5. Cryptogenic
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree