Todd R. Lefkowitz
![]()
29: Entrapment Neuropathies
![]()
PATIENT CARE
GOALS
Provide patient care that is compassionate, appropriate, and effective for the treatment of the adult with an entrapment neuropathy.
OBJECTIVES
1. Describe the key components of the assessment of the adult with an entrapment neuropathy.
2. Discuss the long-term implications of entrapment neuropathies.
3. Assess the impairments, activity limitations, and participation restrictions associated with entrapment neuropathies.
4. Describe potential injuries associated with entrapment neuropathies.
5. Formulate the key components of a rehabilitation treatment plan for the adult with an entrapment neuropathy.
Examination of a patient with a peripheral entrapment neuropathy begins with a thorough history. The physician should inquire about dysesthesias or weakness in the distribution of the peripheral nerve involved, as well as any symptoms outside of the peripheral nerve’s distribution if a more proximal lesion (e.g., radiculopathy or plexopathy) is suspected. It is helpful to ascertain if a patient’s symptoms radiate distally from the neck or shoulder or proximally from the wrist or elbow, although pain patterns often overlap, making it difficult for patients and physicians alike to discriminate between two sites of compression. Concomitant shoulder pain should raise suspicion for a shoulder impingement syndrome.
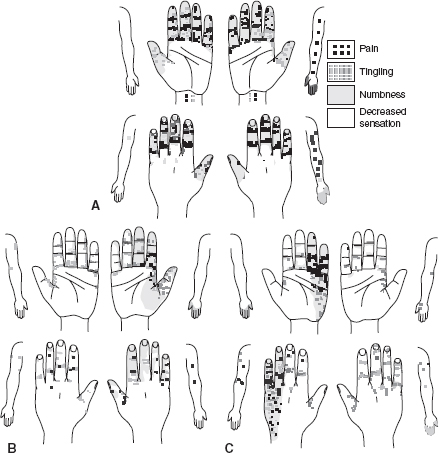
In the upper limb, hand diagrams are useful screening tools for evaluating patients with suspected entrapment neuropathies at the wrist (e.g., carpal tunnel syndrome [CTS]) and elbow (cubital tunnel syndrome [CuTS]) (1,2). The Katz hand diagram (Figure 29.1) is a self-administered inventory where patients’ depiction of pain, numbness, tingling, and decreased sensation can be compared against a symptom classification system of classic, probable, possible, or unlikely CTS. A sensitivity of 80% and a specificity of 90% were reported in one cohort study (3).
The presence of more generalized symptoms in the limbs may suggest an underlying systemic process such as a peripheral polyneuropathy.
A history of recent abdominal, pelvic, gynecologic, or orthopedic surgery should be ascertained in patients with suspected peripheral entrapment neuropathies of the lower limb. Extended lithotomy positions and anterior approaches to hip replacement have been associated with femoral neuropathies at the inguinal ligament (4,5). The obturator nerve and, less commonly, the common peroneal nerve can be injured in the lithotomy position (6). Workers who habitually squat or kneel (e.g., carpenters or farm workers) may preferentially injure the peroneal nerve at the fibular head.
Physical examination of persons with distal entrapment neuropathies of the upper limb should incorporate sensory testing of dually innervated digits (e.g., ring finger and thumb) to help discriminate median from ulnar nerve and median from radial nerve involvement. Two-point discrimination and monofilament testing are commonly used modalities to assess for sensory loss. Atrophy of the thenar eminence, hypothenar eminence, or intrinsic hand muscle weakness should be documented if present. Muscle stretch reflexes should remain unaffected in the absence of a cervical radiculopathy or brachial plexopathy. The Tinel test has historically been used to elicit paresthesias with varying degrees of sensitivity and specificity (7). Other provocative tests such as Phalen’s and the elbow hyperflexion tests have been used to reproduce symptoms suggestive of CTS and ulnar neuropathy at the elbow, respectively.
Sensory testing of patients with suspected distal entrapment neuropathies of the lower limb may be confounded by the presence of a peripheral polyneuropathy, especially in those individuals with a history of diabetes mellitus, chemotherapy (e.g., vincristine, cisplatin), or other toxin exposure (e.g., EtOH). Clinical documentation of muscle weakness is paramount in preparing for the electrodiagnostic (EDX) evaluation. Examination of muscles that have the same spinal nerve root innervation but different peripheral nerve innervation may help distinguish a peripheral entrapment neuropathy from a lumbosacral radiculopathy or plexopathy.
FIGURE 29.1 The Katz Hand Diagram
Source: Adapted from Katz and Stirrat (1990). Copyright 1990, with permission from Elsevier.
Patients with CTS or ulnar neuropathy in their dominant hand may complain of bothersome paresthesias which impair certain work activities requiring fine motor skills (e.g., typing, driving, and electrical work). Ulnar neuropathy, in particular, may be associated with decreased grip strength as the ulnar part of the hand provides a powerful grip. These patients may require written work restrictions from the physician if symptoms are thought to endanger the safety of the patient or those under his or her care. The physician is well advised to image the cervical spine in patients whose hand symptoms are accompanied by new-onset gait disturbance or urinary incontinence to rule out symptomatic cervical stenosis with spinal cord compression.
The physician should counsel patients on how best to modify their work activities such that vibratory and mechanical forces are reduced; however, patients are ultimately responsible for communicating these recommendations directly to their employers (8). This may prove impractical, however, as employers may not allow certain workers with hand-intensive jobs to return to the workplace with restrictions in place in the absence of available light-duty positions.
A trial of non-operative therapy may be attempted in patients with mild-to-moderate symptoms prior to surgical consideration. A referral to an occupational therapist or a certified hand therapist for neurodynamic mobilization exercises or carpal bone mobilization exercises is often prescribed, although the evidence supporting its use is very limited (9). Static splinting of the wrist in persons with CTS may provide relief of symptoms, but evidence is lacking regarding design, wearing regimen, and efficacy compared to other non-surgical treatments (10). Dynamic splinting of the elbow in persons with CuTS may be excessive as a padded elbow sleeve is often sufficient to protect the ulnar nerve at the medial elbow.
There is poor quality evidence from very limited data which suggests that therapeutic ultrasound is more effective than placebo for short- or long-term symptom relief in persons with CTS (11). Likewise, the evidence supporting low-level laser therapy in CTS is also limited (12). Pooled data from four trials found significant short-term benefit from a 2-week course of oral steroids in patients with CTS (13). Compared to placebo, however, patients prescribed diuretics or nonsteroidal anti-inflammatory drugs (NSAIDs) did not demonstrate significant improvement (13).
Roughly 50% of patients with mild CTS may experience pain relief for up to 15 months after a single corticosteroid injection into the carpal tunnel despite earlier reports of no increased benefit after 1 month (14,15). The safest approach for a carpal tunnel injection remains controversial. Historically, physicians used the palmaris longus (PL) tendon as an anatomical landmark to guide their injections. This approach, however, risks injury to the median nerve and inadvertent injection of the nearby ulnar artery. The median nerve was found to extend ulnarly beyond the PL tendon in 88% of hands with CTS in one series (16). As such, some authors advocate injecting through the flexor carpi radialis tendon as the safest route of administration despite the theoretical risk of tendon rupture (17). Ultrasound-guided injections have since been recommended to avoid nerve, tendon, and arterial injuries (17).
Follow-up care of patients with peripheral entrapment neuropathies is necessary, especially after decompression surgery. The recurrence rate of CTS ranges from 3% to 25% with persistent symptoms seen in up to 95% of patients (18–20). Factors associated with poorer outcomes following a carpal tunnel decompression include early symptomatic recurrence, diabetes mellitus, obesity, cervical spine pathology, involvement of the non-dominant hand, and intraoperative scar tissue or fibrosis (21). Recurrence rates of 10% to 13% have been reported following open cubital tunnel release surgery (22,23).
Patients presenting with symptomatic recurrences of a peripheral entrapment neuropathy should be counseled on adopting healthy lifestyle modifications to reduce the burden of systemic disease (e.g., diabetes, thyroid, EtOH consumption) that may be stressing their peripheral nervous system. Consideration should be given to a trial of a nerve membrane stabilizing agent (e.g., gabapentin, pregabalin), although the available literature on these medications primarily reflects experience with patients who have painful diabetic peripheral neuropathy or postherpetic neuralgia and not necessarily peripheral entrapment neuropathies (24).
MEDICAL KNOWLEDGE
GOALS
Demonstrate knowledge of established and evolving biomedical, clinical, and epidemiological sciences pertaining to entrapment neuropathies, as well as the application of this knowledge to guide holistic patient care.
OBJECTIVES
1. Describe the epidemiology, anatomy, physiology, and pathophysiology of entrapment neuropathies.
2. Discuss the common types of entrapment neuropathies and their characteristics.
3. Identify the pertinent laboratory and diagnostic studies important in the evaluation of entrapment neuropathies.
4. Review the treatment of common entrapment neuropathies.
CTS is the most commonly reported nerve compression syndrome followed by ulnar neuropathy at the elbow; however, ulnar neuropathy is only 1/13 as common as CTS (25). CTS affects 3 out of every 10,000 full-time workers according to 2004 National Institute of Occupational Safety and Health data, with a higher prevalence among electrical assembly, food packing/processing, and poultry workers (8). The loss of future earnings of workers with CTS has been estimated to range from $45,000 to $89,000 in one study of workers in Washington State (26).
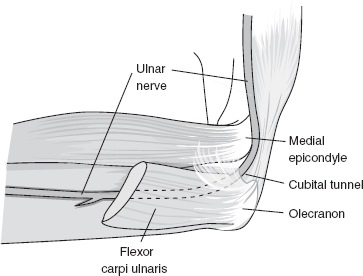
FIGURE 29.2 The Cubital Tunnel
Source: Adapted from Cuccurullo SJ. In: Physical Medicine & Rehabilitation Board Review, 2nd ed. Demos Medical Publishing; 2010.
Compression of the median nerve may result in focal demyelination with or without ischemic changes (27). The ulnar nerve is vulnerable to mechanical compression as it passes between the medial epicondyle of the humerus and olecranon process of the ulna. Repetitive flexion and extension of the elbow can predispose to a traction injury of the nerve as it traverses beneath the proximal edge of the flexor carpi ulnaris (FCU) (Figure 29.2) aponeurosis and the arcuate ligament (e.g., humeroulnar arcade), causing CuTS.
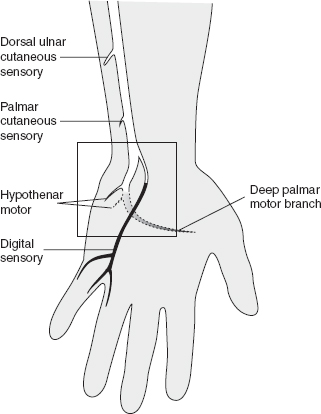
Injuries to the ulnar nerve in the vicinity of the Guyon’s canal are exceedingly rare but may be seen in cyclers (e.g. cycler’s palsy) or those with space-occupying lesions (e.g., ganglion cysts) of the wrist (Figure 29.3).
Distal ulnar nerve entrapments have been previously described by Shea (28).
The existence of a distal entrapment neuropathy with a concomitant cervical radiculopathy (e.g., double crush syndrome) remains controversial. Proponents of this theory argue that a proximal nerve lesion increases the likelihood of a distal entrapment neuropathy by decreasing net axoplasmic flow to an already compromised distal nerve segment, resulting in further axonal damage (29). The median nerve’s extensive root innervation from C6 through T1 may be responsible for the proximal radiation of pain into the upper limb often reported by patients with CTS. Whether this phenomenon represents retrograde degeneration of distal median nerve axons from an initial compressive lesion at the wrist or concomitant compression and sensitization of the dorsal root ganglion in the cervical neural foramen is still unclear.
Peroneal neuropathy at the fibular head is the most common entrapment neuropathy in the lower extremity. The peroneal and tibial divisions of the sciatic nerve run as distinct nerve bundles that do not exchange branches in the posterior thigh. After innervating the short head of the biceps femoris (SHBF) muscle, the peroneal nerve wraps around the fibular head and neck where it closely adheres to the periosteum, making it vulnerable to compression and trauma (Figure 29.4).
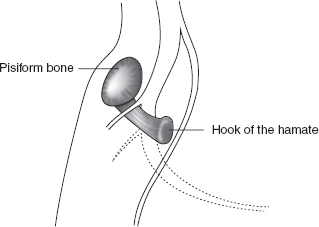
FIGURE 29.3 Anatomy of the Ulnar Nerve at the Wrist
Source: Adapted from Cuccurullo SJ. Physical Medicine & Rehabilitation Board Review, 2nd ed. Demos Medical Publishing; 2010.
The larger fascicles of the peroneal nerve are surrounded by less supportive epineural tissue rendering them relatively intolerant to mechanical compression compared to the tibial division of the sciatic nerve (30). In addition to the more common causes of peroneal neuropathy, severe flexion and inversion ankle sprains can cause traction injuries to the nerve from tearing of the vasa nervosum (31).
The incidence of sciatic neuropathy has been reported to range from 0.05% to 1.9% (32). In patients undergoing traditional hip replacements (via a posterolateral approach), the incidence may be higher with electromyographic evidence of neurologic injury reported to be as high as 70% (33).
Entrapment of the sciatic nerve by the piriformis muscle (e.g., piriformis syndrome) is relatively uncommon despite its widespread use in clinical practice for diagnosing patients with buttock and proximal posterior thigh pain (Figure 29.5). The mean diameter of the piriformis muscle as measured in one cadaveric study was approximately 6.3 mm as compared to 19 mm (~¾ inch) of the sciatic nerve, making true entrapment unlikely in the absence of anatomic variants (34). Most commonly, the sciatic nerve passes undivided below the piriformis muscle, but divisions of the nerve may pass between and below the muscle or between and above the muscle (35,36).
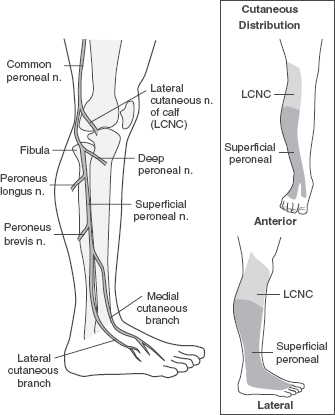
FIGURE 29.4 The Superficial Perineal Nerve
Source: Adapted from Cuccurullo SJ. Physical Medicine& Rehabilitation Board Review, 2nd ed. Demos Medical Publishing; 2010.
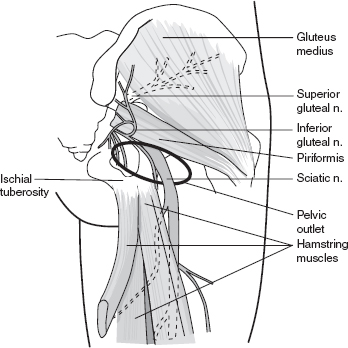
FIGURE 29.5 Piriformis Syndrome
Source: Adapted from Cuccurullo SJ. Physical Medicine & Rehabilitation Board Review, 2nd ed. Demos Medical Publishing; 2010.
The performance of needle electromyography (EMG) in patients with suspected CTS remains at the discretion of the examining physician. It can be used to document axonal loss in those patients with thenar muscle atrophy and decrements in the median compound muscle action potential (CMAP) amplitude in the palm. An abnormal median sensory or motor latency or amplitude, however, does not predict abnormal needle EMG findings (41). Needle EMG should be included as part of the EDX evaluation of patients whose differential diagnosis includes cervical radiculopathy and brachial plexopathy.
In patients with CuTS, there is a 25% chance of finding an abnormality on EMG in the ulnar-innervated forearm muscles compared with a 70% chance of finding an abnormality in the ulnar-innervated intrinsic hand muscles due to the topography of the nerve fascicles traversing the retrocondylar groove (42). Sampling the medial half of the flexor digitorum profundus (FDP) muscle may be of higher diagnostic yield than sampling the FCU muscle, as the branch of the ulnar nerve to the FCU traverses the cubital tunnel in only 50% of individuals (42). A dorsal ulnar cutaneous (DUC) NCS can also help localize a lesion to the cubital tunnel as this nerve does not pass through the Guyon’s canal.
A sciatic neuropathy is a relatively uncommon diagnosis in the EMG laboratory. An absent sural sensory nerve action potential (SNAP) and normal needle examination of the lumbar paraspinal muscles places the suspected lesion distal to the dorsal root ganglion, effectively ruling out a lumbosacral radiculopathy. If the gluteus medius and vastus muscles are normal and the saphenous SNAP is intact, then the lumbar plexus can be considered intact. If there are neuropathic motor units in the external hamstrings and absence of neuropathic motor units in the SHBF muscle, then the most affected portion of the sciatic nerve is proximal to the SHBF.
The EDX evaluation of patients with a suspected entrapment neuropathy of the peroneal nerve is helpful in localizing the lesion above or below the knee. Motor conduction studies to the extensor digitorum brevis (EDB) muscle should be performed. If the EDB muscle is atrophied or a response is difficult to obtain, then recording from the tibialis anterior muscle may be used instead. An accessory peroneal nerve should be suspected if, during routine peroneal NCS to the EDB, the CMAP amplitudes obtained at the fibular head and popliteal fossa are greater than the CMAP amplitude obtained at the ankle. A response obtained by stimulating posterior to the lateral malleolus confirms the diagnosis. Needle EMG of the SHBF can further localize the lesion as the SHBF is the only peroneal-innervated muscle above the knee. Denervation potentials in the SHBF muscle may suggest a sciatic neuropathy if needle EMG of the lumbar paraspinals and gluteal muscles is normal.
An ultrasound examination of the median nerve can complement the EDX survey in cases where the diagnosis of CTS is equivocal. Approximately 10% to 15% of patients with a clinical diagnosis of CTS will have normal NCS, reflecting a sensitivity of 85% to 90% (42). In the early stages of the disease, morphologic changes of the median nerve do not occur; however, a normal ultrasound examination in a patient with clinical symptoms suggestive of CTS does not preclude the diagnosis. As the disease progresses, flattening of the median nerve may be seen at the distal part of the carpal tunnel with enlargement of the nerve at the proximal portion of the tunnel at the level of the scaphoidpisiform (43).
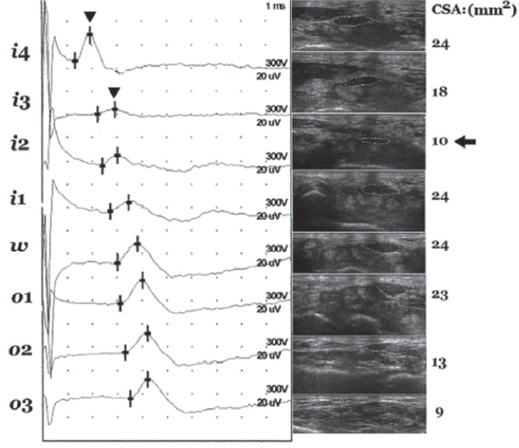
An 8-point ultrasonographic “inching test” measuring the cross-sectional area (CSA) of the median nerve across the wrist has been shown to positively correlate with NCS severity and duration of CTS symptoms (44) (Figure 29.6).
The area 2 cm distal to the distal wrist crease (location i2) had the smallest measured median nerve CSA and is thought to be the most likely site of entrapment in cases of idiopathic CTS (44) (Figure 29.7).

FIGURE 29.6 The eight points (i4, i3, i2, i1, w, o1, o2, and o3) for recording in both the “inching test” and ultrasonography; i4, i3, i2, i1 represent levels at 4, 3, 2, and 1 cm distal to the wrist crease in the inlet of the carpal tunnel; w is at the level of the wrist crease; and o1, o2, and o3 levels at 1, 2, and 3 cm are proximal to the wrist crease in the outlet of the carpal tunnel.
Source: Copyright ©2012 Chen et al.; BioMed Central Ltd.
FIGURE 29.7 An example of “positivesite” between i4 and i3 corresponding to the relatively smaller cross-section area (CSA) at i2. The peak latencies (arrowhead) at i4 and i3 are 1.9 ms and 2.9 ms, respectively, and the difference between them is 1.0 ms, i.e. >0.4 ms. The CSA measured ati2 (arrow) is smaller than those measured at nearby levels. Markers of the 8-point: i4, i3, i2, i1, w, o1, o2, and o3.
Interestingly, longer duration of CTS symptoms correlated with a larger measured median nerve CSA, a finding that has not been previously reported. Diabetic patients with CTS also have larger measured median nerve CSA (45).
Ultrasonographic examination of patients with CuTS may also be performed to complement the EDX survey. Cut-off values for the CSA of the ulnar nerve at the level of the medial epicondyle vary from greater than or equal to 7.5 to 7.9 mm2 (43).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree