Navdeep Singh Jassal
Dayna McCarthy
Jennifer Schoenfeld
Matthew Shatzer
![]()
22: Spasticity
![]()
PATIENT CARE
GOALS
Provide competent patient care that is compassionate, appropriate, and effective for the evaluation, treatment, education, and advocacy for patients with spasticity-associated problems across the entire spectrum of care, and the promotion of health.
OBJECTIVES
1. Describe the key elements of the history and pertinent physical examination of the patient with spasticity.
2. Discuss the impairments, functional limitations, and activity limitations relevant to spasticity.
3. Describe the impact of spasticity on work, school, and community activity.
4. Propose the long-term consequences of spasticity (e.g., contractures, skin breakdown, etc.).
5. Identify potential injuries associated with spasticity (e.g., falls).
6. Identify the psychosocial and vocational implications of the patient with spasticity and strategies to address them.
7. Formulate the key components of a rehabilitation or treatment plan for the patient with spasticity (e.g., stretching program, modalities, etc.).
Clinical evaluation of the patient presenting with muscle overreactivity first and foremost requires an in-depth understanding of the patient’s underlying condition (i.e., spinal cord injury [SCI], traumatic brain injury [TBI], cerebral palsy [CP], stroke) as this will help guide the physician’s treatment plan. Long-term treatment goals and functional goals are heavily influenced by whether the underlying pathology is progressive versus static, whether the condition is acute or chronic, as well as past treatments utilized and their outcomes. Other etiologies of increased tone such as rigidity, catatonia, gegenhalten, or contractures (1) must also be ruled out. Identification of potential triggers such as pressure ulcers, ingrown toenails, catheter obstruction, kidney stones, urinary tract infection (UTI), deep venous thrombosis (DVT), heterotopic ossification, constipation/impaction, sepsis, and/or fractures commonly leads to increased tone and should be identified during initial evaluation. Improper body positioning can often contribute to worsening spasticity and contracture. Postures that have a negative impact include a scissoring posture (bilateral hip extension, adduction, internal rotation), windswept position (hip flexion, abduction, external rotation on one side and relative hip extension, adduction, and internal rotation on the other), and frog-leg position. Identification of a clinical pattern (e.g., location, duration, frequency), the presence of pain, the patient’s ability to control involved muscle groups, and the functional impact are also included in comprehensive history pertaining to spasticity.
When approaching the physical examination, there are several key features that help the clinician identify spasticity. Velocity dependence is a requirement in the diagnosis of spasticity; this condition is defined as a velocity-dependent increase in tone. Currently, the Modified Ashworth Scale (MAS) that tests resistance to passive movement about a joint with varying degrees of velocity and is scaled from 0 to 4—with 0 indicating no tone and 4 indicating rigidity—is the most widely utilized scoring system to classify tone. In addition, there is the Tardieu scale, which quantifies muscle spasticity by assessing the response of the muscle to stretch applied at specified velocities. The MAS, in addition to motor strength testing and thorough ROM testing, comprises the most important clinical examination findings for spasticity. Special signs that may be present on examination are the “Clasp-knife” phenomenon as well as the “stroking effect,” which are not pathognomonic but are commonly elicited. Spasticity more commonly affects antigravity muscles in the upper extremity. The most common patterns of upper motor neuron dysfunction include adducted/internally rotated shoulder, flexed elbow, pronated forearm, bent wrist, clenched fist, thumb in palm, and an intrinsic plus hand. In the lower extremity most commonly seen patterns include flexed hip, scissoring thighs, stiff knee, flexed knee, equinovarus foot with curl or claw toes, valgus foot, and hyperextended first toe.
Limitations as a result of spasticity are dependent on severity and muscle involvement. At the very least it can cause discomfort and pain. Because it limits joint range of motion (ROM), it has the potential to limit mobility and produce deformities if left untreated. Most individuals will require orthosis for independent mobility. If the spasticity is severe enough, mobility may be limited to a wheelchair. Limited mobility may also result in pressure changes that can lead to skin breakdown. Spasticity can present challenges in respect to hygiene maintenance, bowel/bladder care, and sexual relationships, as the axilla, hands, and genitals can be difficult to access. It is important to understand that spasticity is not always detrimental. For instance, spasticity in trunk muscles may help with transfers; hip and knee extensor spasticity facilitates standing, transfers, and ambulation; soleus spasticity helps children with CP toe off; and finger flexor spasticity may allow people to manage utensils and self-care items.
Spasticity can substantially impact the quality of life in those affected. In surveys of patients’ perceptions of problems, spasticity has been consistently identified in the top three to five life concerns (2). The Patient Reported Impact of Spasticity Measure (PRISM) was created to define and measure extents to which quality of life is affected in SCI patients with spasticity. The 7 factors assessed are social avoidance/anxiety, psychological agitation, daily activities, need for assistance/positioning, positive impact, need for intervention, and social embarrassment (3). Although this scale is used in a specific subset of patients with spasticity, it can be extrapolated for the spastic population and can offer insight into common difficulties that they face in interactive environments.
Left untreated, spasticity can lead to contractures that are difficult to correct and make self-care, hygiene, mobility, and transfers extremely difficult for both patient and caregiver. Over time asymmetric pull of overactive muscles can cause deformities and alter posture. Positioning and pressure relief are constant considerations as there is a high prevalence of skin breakdown in this patient population. Most common areas of skin breakdown include hands, axilla, elbows, genital area, sacrum, and ischium.
The most common injuries seen as a result of spasticity are those related to impaired mobility, such as falls, and pressure ulcers. When a patient is nonambulatory secondary to spasticity he or she has a higher propensity to sustain fractures especially in long bones. Hip dislocation may also occur in those that have hip joint involvement that is severe.
The severity of spasticity will guide the treatment plan, and rehabilitation must always be included. When constructing a therapy plan for spasticity, the goal is to reduce pain, regain function, and/or preserve function. This is accomplished by focusing on aggressive passive ROM, with a goal of 2 hours of stretching per day. In cases of focal spasticity, splinting/orthosis may also be indicated. There are several different approaches utilized by physical therapists to treat spasticity; most literature exists regarding techniques for stroke patients. The Bobath method aims to reduce spasticity through attention to trunk posture and controlled muscle stretch of the limbs. The Brunnstrom method advocates techniques to promote activity in weak agonists by facilitating contraction of either corresponding muscles in the unaffected limb or proximal muscles on the paretic side. No solid evidence exists for the use of modalities for spasticity; however, there is a small body of evidence that electrical stimulation techniques, although used for movement loss related to muscle paresis, may prove to be a useful adjunct to other treatments such as Botox (4).
MEDICAL KNOWLEDGE
GOALS
Demonstrate knowledge of established and evolving biomedical, clinical epidemiological, and sociobehavioral sciences pertaining to spasticity, as well as the application of this knowledge to guide holistic patient care.
OBJECTIVES
1. Describe the epidemiology of spasticity.
2. Describe the anatomy, physiology, and pathophysiology of spasticity.
3. Review the treatment options and rehabilitation components in spasticity.
4. Recognize the complications and red flags associated with spasticity.
Spasticity is a condition seen in upper motor neuron disorders (UMNDs). Conditions that include spasticity as a clinical feature include but are not limited to SCI, stroke, multiple sclerosis, amyotrophic lateral sclerosis, hereditary spastic paraparesis, and CP. It is rare for spasticity to occur on its own; rather, it occurs as one of the positive components of UMND, which also include exaggerated tendon reflex, clonus, spastic dystonia, increased tone, released reflexes, and Babinski sign. UMNDs also comprise negative components which include loss of motor control, loss of selective motor control and dexterity, slowed movements, and spastic cocontractions (1).
To understand the pathophysiology of spasticity, it is important to first understand normal anatomy and physiology of motor control. The motor system is a highly complex and integrated system that requires input and constant feedback. At the most distal aspect of the system there is the motor unit that comprises three main fibers, Type 1 (slow, aerobic), Type II (fast, anaerobic), and mixed (both Type I and Type II). When working properly, motor units fire with the coordination of an agonist and antagonist system, with normal patterns of recruitment (5). A feedback loop utilizes input regarding muscle length, muscle tension, joint position, and velocity. Muscle spindles are a major contributor to the feedback loop (Figure 22.1A, B). Attached to the muscle mass and containing both Ia and II fibers, the spindle is responsible for relaying information regarding position and rate of change of the muscle throughout ROM. The spindle also contains the gamma motor neuron, which is responsible for maintaining proper tension of the spindle. Within the muscle tendon exists the Golgi tendon organ. Comprised of Ib fibers, it is responsible for limiting muscle contraction to prevent musculotendinous injury by initiating antagonist and inhibiting agonists. Moving proximally, spinal interneurons (Ia, Ib, Renshaw, propriospinal) play a large role in motor control. Ia and Ib interneurons work to facilitate both the muscle spindle and the Golgi tendon organs, respectively. Renshaw cells receive input directly from the alpha motor neuron allowing for direct cessation of agonist activity by directly engaging the alpha motor neuron. They also promote antagonist’s function by way of the antagonist’s Ia interneuron. Supraspinal stimuli are diverse and complex. The corticospinal tract is derived from extrapyramidal cells from the prefrontal region, the supplementary motor region, the cingulate gyrus, and the postcentral gyrus of the parietal lobe. Extensor pathways of the brain are the pontine medial reticulospinal and lateral vestibulospinal pathways. The pontine system facilitates the alpha and gamma motor neurons of the extensors of the limb muscles with some input into the system from the sensorimotor cortex. The lateral vestibular is found in the ventromedial portion of the cord and terminates at the spinal cord motor neurons. Stimulating this tract affects the motor neurons of the flexor muscles differently from the extensors, with the alpha and gamma motor neurons of the flexors inhibited and those of the extensors facilitated. The nucleus of the cerebellum also has an excitatory influence on extensor pathways (6). There are numerous pathways that facilitate flexion, whereas extensor inhibition occurs mainly in the medullary lateral reticular formation (MLRF). The cortex facilitates MLRF’s action, and cortical injury can lead to net overactivity of the lower extremity extensor system. The MLRF demonstrates its effect through its connections to the motor neurons, type Ia interneurons, and type Ib system. In cats the corticospinal, corticoreticulospinal, and corticorubrospinal tracts all show significant flexor facilitation. Through interneurons, the corticorubrospinal tract excites flexor motor neurons and inhibits extensors. In addition, the medullary reticulospinal tract is a predominant part of a largely flexor-oriented system (7).
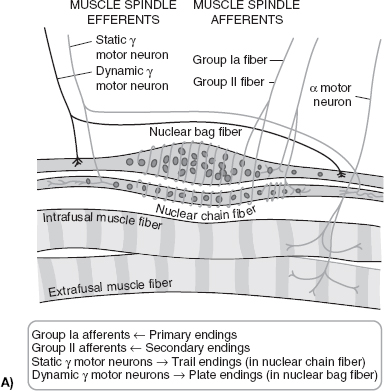
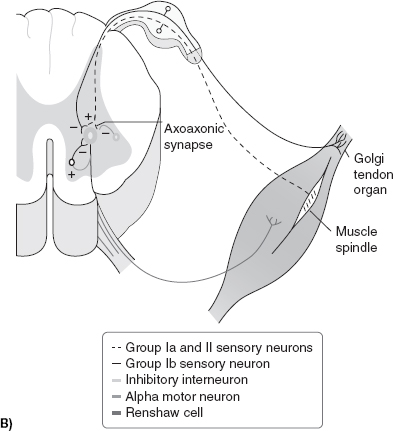
FIGURE 22.1 (A): Nuclear bag and nuclear chain fibers of the muscle spindle. (B): Influences on the stretch reflex. (Redrawn from Braddom, RL. Physical Medicine & Rehabilitation, 4th ed., Chapter 30, Philadelphia, PA: Elsevier; 2011.)
Spasticity is defined clinically as a motor disorder characterized by velocity-dependent increase in stretch reflexes with exaggerated tendon jerks, resulting from hyperexcitability of the stretch reflex. Although there is no definitive consensus on the pathophysiology of spasticity, the most commonly utilized theory is that a lower threshold exists for motor neuron response to stretch. This lower threshold is coupled with long discharges that result in an alteration of the balance between inhibitory and excitatory inputs to motor neurons, with the excitatory component being more frequently activated. Some have expressed the belief that the ionic properties of the membrane itself are changed as well. Other proposed theories involve central collateral sprouting, presynaptic disinhibition, and denervation hypersensitivity, as well as neurotransmitters serotonin and substance P malfunction (8).
There are a variety of treatment options available for spasticity. The clinician must be aware that increased tone is not always detrimental, in many cases the existence of tone allows for functional capacity that wouldn’t be obtainable in its absence. As such, spasticity requires consistent monitoring and feedback by both the patient and the treatment team to maintain the appropriate balance for optimized function. Persistent neglect of this condition will lead to progression of spasticity and eventually rigidity and contractures. It is also a condition that fluctuates in severity based on nociceptive, visceral, or somatic stimuli. Depending on the primary pathology it may also worsen as the disease progresses. The goal of the provider when treating spasticity may include pain reduction, decreased spasms, facilitation of orthotic management, ease of care, changing positioning for pressure relief, release inhibition of antagonists, as well as improvement in hygiene, transfers, activities of daily living (ADLs), and mobility. As with most treatments it is always advisable to start conservatively, especially in the acute phase of the primary injury because more aggressive interventions may negatively impact recovery. As discussed previously, the mainstay of conservative management is constant stretching, minimum of 2 hours/day, to prevent progression of tone and to maintain ROM. In cases of focal spasticity, splinting/orthosis may also be indicated. There are several different approaches utilized by physical therapists to treat spasticity, and most literature exists regarding techniques for stroke patients. The Bobath method aims to reduce spasticity through attention to trunk posture and controlled muscle stretch of the limbs. The Brunnstrom method advocates techniques to promote activity in weak agonists by facilitating contraction of either corresponding muscles in the unaffected limb or proximal muscles on the paretic side. Other modalities that haven’t been strongly studied but are still utilized by therapists include cryotherapy, electrical stimulation, splinting/casting, positioning, vibration therapy, and relaxation techniques.
If spasticity remains detrimental after conservative treatment and removal of inciting factors, then pharmacological management may be indicated. When considering oral medication for the treatment of spasticity the functional impairment and configuration of involved muscles must be factored into the choice of pharmacologic management. Generally speaking, individuals with processes that result in diffuse spasticity (i.e., SCI, MS) will respond to oral agents whereas those with focal spasticity will not. Current oral medications indicated for the treatment of spasticity focus on enhancement of segmental inhibition via GABA (baclofen), modulating the monoamines (tizanidine), alteration of ion channels (dantrolene, benzodiazepines, clonazepam, gabapentin, pregabalin, topiramate, vigabatrin, lamotrigine, riluzole, clonidine, cyproheptadine, and chlorpromazine), and inhibition of excitatory amino acids (orphenadrine, memantine, carisoprodol, and cannabinoids). Although the list of potentially beneficial oral agents is extensive, there are several that are more commonly used and therefore warrant more detailed descriptions. These include baclofen, diazepam, tizanidine, dantrolene, and clonidine.
Baclofen is a GABA agonist that is particularly useful for spasticity secondary to SCI and MS. Dosing is usually started at 5 mg twice daily and increased to three times daily with a maximum daily dose of 80 mg. The side-effect profile is somewhat extensive and includes but is not limited to a lower seizure threshold, sedation, weakness, GI symptoms, tremor, insomnia, and confusion. It is also important to note that baclofen should never be withdrawn suddenly and must be tapered off to prevent seizures.
Diazepam (Valium) has demonstrated benefits in those with SCI- and MS-related spasticity by facilitating GABA’s effect on receptors. It is not indicated for those with TBI secondary to its negative effects on attention and memory. The starting dose is usually 4 mg at bedtime or 2 mg twice daily with a maximum dose of 60 mg/day. Side-effect profile most commonly includes sedation, memory impairment, and decreased REM sleep.
Tizanidine (Zanaflex) is a centrally acting alpha-2 adrenergic agonist with the typical side-effect profile of sedation, hypotension, dry mouth, bradycardia, dizziness, flushing, and liver toxicity. For the latter, baseline liver function tests (LFTs) are indicated before starting this medication as well as consistent LFT monitoring. Studies have shown tizanidine to be as effective as baclofen and diazepam in treating spasticity in SCI, MS, and TBI, as well as better overall tolerability. The starting dose is 2 to 4 mg at night, which may be increased to a maximum of 36 mg/day as tolerated.
Clonidine (Catapres) has the same mechanism of action as tizanidine with the same side-effect profile with a more profound effect on blood pressure leading to hypotension and syncope, as well as ankle edema and depression. This medication is most commonly prescribed in a transdermal form of 0.1 mg/week with a maximum dose of 0.3 mg/week. The oral form is dosed at 0.05 mg twice daily with a maximum dose of 0.4 mg/day.
Dantrolene, most commonly known for its treatment of malignant hyperthermia and neuroleptic malignant syndrome, has classically been used for spasticity of central origin based on its peripheral mechanism of action (blocks Ca++ release from sarcoplasmic reticulum of striated muscle). As with tizanidine this medication requires monitoring of LFTs as there is a high risk of liver toxicity. Other side effects include weakness, fatigue, paresthesias, diarrhea, nausea, and vomiting. The starting dose is 25 mg twice daily with maximum dose of 400 mg/day.
Individuals with more focal spasticity (i.e., stroke, TBI) respond favorably to injectables. Most commonly used particulates for chemodenervation are botulinum toxin type A and phenol. It should be noted that phenol is not recommended for treatment of upper limb spasticity (4). Phenol has the ability to block spasticity for months to years and is most commonly used in concentrations of 2% to 7%. Complications of phenol blocks include dysesthesias, muscle pain, muscle weakness, transient edema, DVT, skin sloughing if injected superficially, and serious systemic reactions if injected intravascularly.
The neurotoxin derived from Clostridium botulinum bacteria has seven serotypes of which only A (Botox, Dysport, Xeomin) and B (Myobloc) are available in the United States. When injected, the toxin is taken up by the nerve terminal where it prevents exocytosis of acetylcholine into the nerve terminal cleft. The effects of botulinum toxin last anywhere from 2 to 6 months with peak effect occurring around 4 to 6 weeks. Dosing is different depending on type. Type A dosing is 25 to 200 units per muscle, with a documented maximum dose of 400 units, although much higher doses have been administered without adverse effects. Type B starting dose is 10,000 units. The protocol for both forms is spacing of 3 months between injections and injections are most commonly administered using EMG guidance to ensure correct anatomical position of targeted muscle. Contraindications include known sensitivity, myasthenia gravis, Lambert-Eaton syndrome, motor neuron disease, and concomitant treatment with aminoglycoside or spectinomycin antibiotics. The side-effect profile includes weakness, ecchymosis, flu-like syndrome, dysphagia, nerve trauma, pain/soreness, antibody formation resulting in ineffectiveness of future botulinum toxin injections.
There is an art to the treatment of spasticity and the approach to treatment should always be conservative. There exists the potential for serious adverse events with all of the treatment options discussed. It is imperative that the physician be thoroughly educated and understands the importance of introducing new treatments slowly, as well as closely weighing out the risks/benefits, including costs, before starting new treatments. For instance, it would be negligent for a physician to overdose a patient with Botox leading to weakness, a fall, and a hip fracture.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


