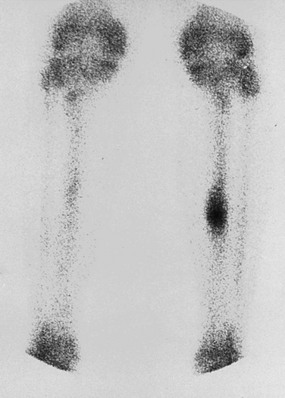
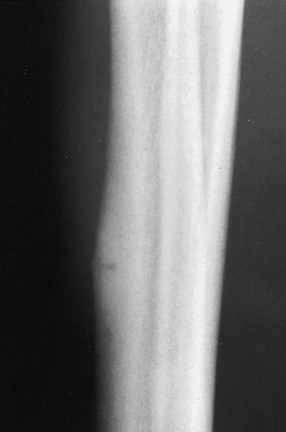
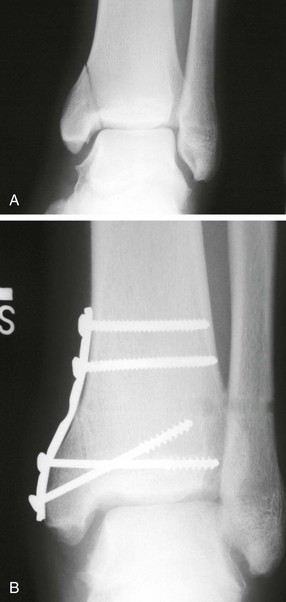
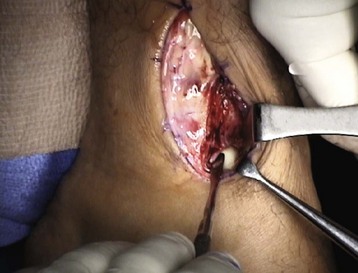
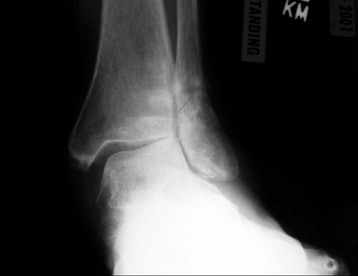
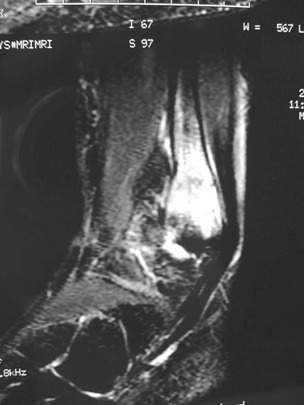
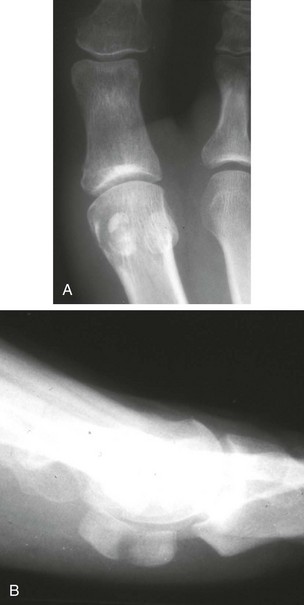
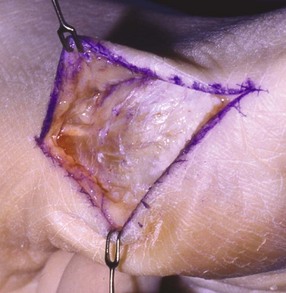
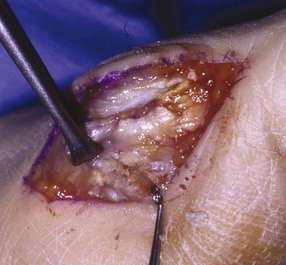
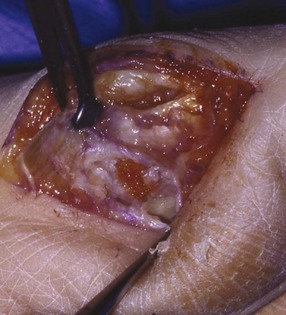
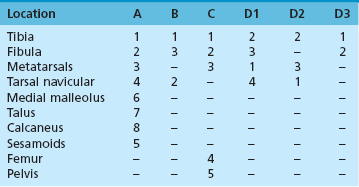
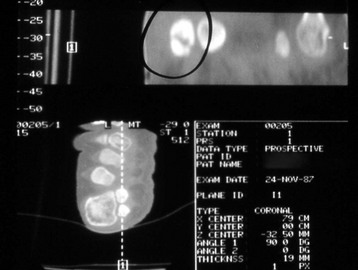
Chapter 31 Stress fractures were first described by the German military surgeon Breithaupt in 1855, before the development of radiography. Kirchner, in 1905, was the first to describe the radiographic findings of this entity, which are preceded by 2 to 3 weeks of clinical symptoms.72 Stress fractures of the foot and ankle are a common clinical finding. The presentation can vary as well as the location. This entity is a dynamic clinical syndrome that is characterized by exertional pain, localized tenderness, and swelling.46 Stress fractures occur as the result of repetitive and prolonged muscle action on bone that fails to accommodate. Daffner24 described a fatigue triad that included activity that was new, strenuous, and repeated. This results in the development of insufficiency of the bone within the ranges of normal or physiologic muscle activity. Recently, there has been attention given to what is termed “stress reaction.” A stress reaction is characterized by normal radiographs with increased uptake seen on magnetic resonance imaging (MRI) without a discrete fracture line. The findings on advanced imaging demonstrate increased marrow edema without cortical thickening, new bone formation, or periosteal reaction.14 Stress fractures of the foot and ankle account for the vast majority of stress fractures. About 80% of these fractures involve the tibia, fibula, metatarsal, or calcaneus (Table 31-1). Other stress fractures are likely underreported, such as those of the navicular and sesamoids. Although some stress fractures only cause frustration from pain and activity modification, others can result in a lifetime of disability. The diagnosis and treatment of each must be individualized. Fractures with certain etiologies require a thorough examination of bone metabolism or biomechanics. This chapter details the various types of stress fractures afflicting the foot and ankle and the treatment options available, with the authors’ recommendations included. Table 31-1 Ranking of Frequency of Stress Fractures in Running Athletes D1, general athletic population; D2, track athletes; D3, distance runners. A, From Baxter DE, Zingas C: The foot in running, J Am Acad Orthop Surg 3:136–145, 1995. B, From Bennell KL, Malcolm SA, Thomas SA, et al: Risk factors for stress fractures in track and field athletes. A twelve-month prospective study, Am J Sports Med 24:810–818, 1996. C, From Renström AF: Mechanism, diagnosis, and treatment of running injuries, Instr Course Lect 42:225–234, 1993. D, From Brukner P, Bradshaw C, Khan KM, et al: Stress fractures: a review of 180 cases, Clin J Sport Med 6:85–89, 1996. The most commonly studied population for the incidence of stress fracture is military recruits. The significant physical requirements, the varying training regimens, and particularly the rapid acceleration of training, makes this population at risk for stress fractures. Milgrom83 studied 295 recruits in the Israeli army during an intensive physical training regimen. The alarming incidence of stress fractures in this young, active group was 31% (91 of 295). Thirty-five percent of the patients were asymptomatic, and 20% were noted to have plain radiographic findings of stress fracture. Fracture frequency by location was as follows: 51% tibial diaphysis, 5% tibial plateau, 21% femur, 13% distal femur, and a very low incidence of tarsal and metatarsal fractures.82,83 The rates of stress fractures in soldiers in the United States has not been as significant as those found by Milgrom; some authors estimate a rate of less than 2%.94 Pester et al94 completed a 4-year study of U.S. military recruits at Fort Dix. The study demonstrated a significant difference in fracture rate by location between the male and female recruits. The male recruits had a predominance of metatarsal stress fractures (66%) and 20% calcaneal fractures. Only 13% of the fractures were located in the leg. The female recruits had a much higher incidence of calcaneal fractures and tibial fractures (39% and 27%, respectively). The metatarsal fracture incidence was lower (39%). The increased calcaneal fracture rate in female recruits may be the result of differences in marching techniques and training. There was no correlation with height, weight, age, or foot type. The female recruits had a two times higher incidence of bilaterality and early occurrence of fractures during their training (first week). Greaney43 has reported that 77% of stress fractures are cancellous and 23% are cortical. There is likely a natural progression from microfractures to stress reactions to frank cortical fracture. Stress fractures develop when the natural response of the skeletal system to loading is overtaxed. It is postulated that stress reactions occur by cyclic loading that does not exceed the ultimate breaking stress or limits of plastic deformation of bone. One possible cause of stress fracture is muscle fatigue, which decreases shock-absorbing capacity of the extremity, and another is repetitive force exerted by a muscle on bone. From a biomechanical standpoint, muscles increase their response to force quicker than bone does. Bone is a dynamic structure and responds to muscle activity. As stress increases, this leads to bone deformation along its elastic range, and the bone returns to its prestress state after the applied force is removed. When the elastic range is exceeded, this causes plastic deformity, which leads to microfractures. These microfractures or cracks can progress to structural failure.24 The diagnosis of a stress fracture rests on a number of factors and includes a spectrum of presenting symptoms and signs. The stress reaction is a prodromal symptom and one the athlete might try to endure or run through. This phase includes a vague discomfort with activity and varying degrees of swelling. There is occasional tenderness over the involved site, which bone percussion can help localize. A one-legged hop test can also elicit pain. Eventually, a fracture occurs if loading is continued. This is because osteoclastic resorption occurs 5 to 14 days after onset, and increased vascularity occurs with endosteal and periosteal callus formation and leads to a weakened bone state. The differential diagnosis at this stage includes tendinitis, periostitis, pes bursitis, compartment syndrome, and gastrocnemius–soleus complex injury.76 Imaging studies confirm the diagnosis of stress-related injuries. Plain radiographs are the mainstay in conventional fracture diagnosis but are often not helpful unless there is cortical disruption. In Matheson’s74 study of 320 stress fractures in athletes, it was noted that the period of pain onset to bony changes was weeks to months (average, 10 to 21 days) and that bone changes may be evident in only 30% to 70% of cases. A technetium-122 (99Tc) bone scan is extremely sensitive, and uptake may be evident within 24 hours after injury. However, this test is not specific. On the other hand, MRI is sensitive and specific, particularly to location. Computed tomography (CT) is helpful only to define a fracture line and to determine whether the fracture is complete or incomplete. The plain radiographic findings include rarefaction of bone, sclerosis, endosteal callus, cortical fissure or crack, and the occasional complete fracture.46 Early radiographic findings include lucency without periosteal reaction or callus. The first sign is focal linear sclerosis that occurs perpendicular to the trabeculae. As this bone enters the healing phase, the appearance of thick laminar periosteal bone is noted.24 Levy described two types of stress fractures.72 The diaphyseal stress fracture is characterized by cortical disruption, followed by dense callus. This is most common in the lesser metatarsals (second through fifth). Fractures in ends of long bones occur in cancellous bone and appear as lines perpendicular to the direction of the applied stress. This type is most common in the calcaneus and the first metatarsal.68 Much attention recently has been give to the use of teriparatide in the treatment of osteoporosis in postmenopausal women. Teriparatide is a recombinant human parathyroid hormone analog that was approved by the Food and Drug Administration (FDA) in 2002 for the treatment of osteoporosis in women who are at high risk for insufficiency fractures. The mechanism of action is by stimulating new bone formation by favoring osteoclasts over osteoblasts. Animal studies have shown an enhanced callus formation increase in strength in healing of tibial fractures.103 The only prospective randomized study to date in humans demonstrating accelerated fracture healing was performed in 2010. In 102 postmenopausal women with distal radial fractures, the teriparatide group had shortened time-to-fracture healing versus placebo. Current ongoing research will further discern the efficacy of this medication in the treatment of stress fractures.9 The use of calcitonin has also been postulated to enhance fracture healing, but current literature has not demonstrated these effects. Nonsteroidal medications can reduce the inflammatory component but might interfere with bone production. Bone stimulation is also without proven benefit in this situation but is reasonable if it is accessible and affordable. Extracorporeal shock wave therapy has been postulated as a future treatment adjuvant. Alvarez et al3 reviewed the use of shock wave therapy in the treatment of proximal metatarsal fractures. Although this was not a comparative study, the authors demonstrated satisfactory healing rates in these fractures without any complications from this adjuvant treatment.3 Most stress fractures heal in 4 to 15 weeks. Once the fracture has healed, shoe modifications with biomechanical correction are necessary, as is the avoidance of a premature return to activity. Nutritional and dietary factors can influence healing in stress fractures. Wentz et al,123 in 2012, studied dietary and training predictors of stress fractures in female runners. Of interest, an important influence on the bone mineral density of these athletes was their milk intake during middle school. The most predictive factors for stress fractures were low bone mineral density, current calcium intake, irregular menstrual cycles, the years of running history, and training on hard surfaces.123 Tibial stress fractures are the most common stress fractures seen in an active population. In military recruits and athletes, it tops the list as the most common location of an acute stress fracture.46,76,82,83 In 1958, Devas29 described the entity of shin soreness, which was analogous to a stress fracture of the tibial diaphysis. Milgrom83 reported on the associated factors predisposing to the development of tibial stress fractures. These factors were examined in soldiers in the Israeli military. Soldiers with narrower tibias had increased incidence of stress fractures compared with those with wide tibias. This relationship was thought to be a factor of the area moment of inertia about the bending axis of the bone. After looking prospectively at risk factors for development of a tibial stress fracture, the moment of inertia, determined on the cross section of the tibia, was a better predictor of stress fracture risk than tibial width.82 The most common location of a tibial stress fracture is along the posteromedial cortex, which may be due to the repeated pull of the gastrocnemius–soleus complex, leading to failure of the bone with high levels of activity.29,30 Ekenman36 specifically looked at the relationship of the posteromedial location of these fractures to the muscle attachments in this region. They found that the strain and pull of the deep plantar flexors and their attachment results in subperiosteal bleeding and can lead to cortical disruption (see also Chapter 26). The primary clinical symptom of tibial stress fracture is pain with activity, particularly near the end of activity. The intensity typically begins as a very mild discomfort that abates with rest. This typically escalates to where the pain is reproduced with shorter distances or a lower level of activity, and the pain persists for a longer time after activity. This prodrome gradually increases over time. It is very rare to have acute-onset pain that does not resolve with short periods of rest. The pain and discomfort can progress to the point that the patient cannot participate in the activity, and training has to be discontinued.27 Physical examination typically reveals tenderness at the midshaft tibial region. The tenderness can be associated with swelling, erythema, and warmth. The tenderness is more commonly located on the medial border of the tibia in this location.29 Percussion can elicit specific tenderness. Radiographs are typically normal in the early stages, but they can demonstrate some periosteal reaction or cortical thickening as early as 2 to 3 weeks from the time of the earliest symptoms. The early stages can show a mild cortical lucency, which later becomes an area of increased density accompanied by a more noticeable periosteal reaction with cortical thickening. 99Tc bone scanning or MRI is very useful in detecting stress fractures in the early or evolving stages (Fig. 31-1). The fracture line, when seen on plain radiographs, usually courses from the posteromedial cortex.29 These posteromedial diaphyseal fractures typically have a more benign course and resolve with conservative measures.56 A more infrequent location of a tibial diaphyseal stress fracture is along the anterior cortex. This is seen as an anterior cortical line, often called a “black line,” which represents a more difficult condition to treat (Fig. 31-2). This anterior stress fracture is believed to indicate or predict the development of symptomatic pseudoarthrosis.97 Stress fracture in this location appears to be associated with jumping activities more than the posteromedial location is.35,37 Treatment of the anterior tibial stress fracture should be undertaken with more vigilance than the typical tibial stress fracture. When an anterior cortical lucency is noted, there is a higher risk of delayed union or of progression to complete fracture.27 Green44 noted a significant nonunion rate in their athletes who had this entity (5 out of 6). Their recommendations included surgical treatment consisting of excision and bone grafting if no healing had occurred by 4 to 6 months from diagnosis. Other treatment options include intramedullary reaming and fixation, drilling of the defect, and electromagnetic bone stimulation.27,97,105 Johansson et al56 reviewed 11 patients with anterior tibial stress fractures. They found that in contrast to the positive results of Rettig105 with conservative treatment plus electromagnetic therapy, their patients failed conservative care (which consisted of 3 months of immobilization) and ultimately required operative intervention. Even in the operative fixation and grafting group, the average time to bone union was approximately 5 months. Theories explaining the difficulty with this fracture location include the relative hypovascularity of this region. Stress fracture of the medial malleolus was first described by Devas in 1975.29 These fractures account for 9% of all foot and ankle stress fractures.104 Shelbourne,112 in 1988, presented the first clinical description of this entity as well as discussion of the results of treatment. The description included tenderness at the region of the medial ankle (often associated with an effusion), the presence of activity-related pain, and often a vertical fracture line from the tibial plafond and medial malleolar junction. This fracture can propagate in a superior and oblique fashion. This fracture has an appearance similar to that of the supination–adduction injury described by Lauge-Hansen.66 It is theorized that the combination of dorsiflexion and rotation can cause excessive or repetitive force transmission to the medial malleolus. Excessive pronation can prevent or limit tibial external rotation and subsequently increases tibial internal rotation. This can lead to microfracture in the region and propagation of failure or fracture vertically at the medial malleolar–tibial plafond junction. Impingement with dorsiflexion of the ankle can also play a role by increasing stresses at the malleolar junction. Premature abutment of the talus to the medial malleolus may create chronic impingement, which can lead to the development of a medial malleolar stress fracture. This anteromedial impingement may be the result of osteophytes on the distal tibia or talar neck (Cam lesion). These lesions need to be addressed at the time of surgical reconstruction if present.59 Plain radiographs are usually normal as late as 2 months after the initial appearance of symptoms.90 Additional useful radiographic studies are 99Tc bone scanning, CT, and MRI. Okada88 was the first to describe the appearance of a medial malleolar stress fracture in a case report in 1995. The T1-weighted MRI image demonstrated a vertical linear zone of decreased signal intensity at the junction of the medial malleolus and the tibial plafond. It is very difficult to determine on MRI whether the fracture line is complete or incomplete because of the extensive signal variation (Fig. 31-3). It is recommended that a CT scan with coronal and sagittal reconstructions be obtained in the event of a positive bone scan or MRI. The CT scan is useful to determine the extent of the fracture and is particularly helpful in preoperative planning to determine the fracture line location as well as the need for adjuvant bone grafting. Orava,90 in following eight patients, noted that after 2 to 3 months of conservative treatment, symptoms resumed with activity. In their series, the average time to healing was 9 months (range, 5 to 17 months) from the onset of symptoms. Three of the eight patients eventually required surgery in the form of drilling of the lesion without internal fixation. Shabat110 reviewed the literature in 2002 and found 23 cases described from 1988 to 2002. The healing rates and treatment were very variable. The approximate time to return to sports activity was doubled in the nonoperatively treated group. The time to complete healing of the fractures ranged from 2 to 8 months. The surgical group averaged 4.2 months, and the conservatively treated group took 6.7 months on average. Surgical treatment ranged from drilling alone to formal fixation with bone grafting (Fig. 31-4). Our treatment protocol includes activity restriction and immobilization with weight bearing to tolerance in the acute setting. The use of rigid immobilization in a cast limits dorsiflexion of the ankle, which prevents possible impingement of the talus on the medial malleolus. Percutaneous screw fixation of incomplete fractures can be justified, but caution is taken to avoid injury to the posterior tibial tendon. If there is evidence of a complete fracture line on plain radiographs or CT scan, surgery is typically recommended. For fracture fixation in cases with a vertical fracture line, buttress (antiglide) plating should be considered. Adjuvant bone graft should also be considered in patients with complete fracture lines. It is critical to assess the anteromedial joint line for areas of bony impingement. If present, debridement of both talar and tibial osteophytes is necessary (Fig. 31-5). In cases with an established nonunion, the authors recommend open reduction and internal fixation, take-down of the pseudarthrosis, and bone grafting. In cases that do not have complete fracture line on MRI and CT scan and that have failed initial conservative measures, fixation without formal bone grafting can be performed. Fatigue or stress fractures of the distal fibula were first reported by Detelfsen (1937) and Burrows17 (1940). These fractures were noted to occur in the distal fibula, ranging 1.5 to 3 inches from the tip of the lateral malleolus.17 Burrows described two distinct patient groups for these fractures. The first is young male runners or skaters. Their fracture location appears to have a stronger correlation with increased activity and occurs at a more proximal location than in the second group. The second patient group was middle-aged women. In this group, the fracture occurred more distally.17,18 The more distal location of the fracture is characterized by a greater amount of cancellous bone when studied in cross section, whereas the proximal location is primarily in dense cortical bone.17 Stress fractures of the fibula are much more common at the distal third. These are seen most often in athletes and military recruits.29,84 Pester94 found an incidence of 1% to 5% in military recruits. The clinical presentation is typically characterized by an abrupt onset of pain, usually within 10 days of the increased activity or alteration of training. There is typically diffuse edema, generalized pain, and tenderness that is often difficult to locate.29 The tenderness, when present, is usually located at the posterolateral border of the fibula approximately 4 to 7 cm from the tip of the malleolus.29 If the presentation is subacute, a common clinical finding is palpable prominence from the abundant callus. Ankle range of motion is usually well preserved.17 Radiographic findings demonstrate the fracture within 3 or 4 weeks of the initiation of symptoms. The characteristic radiographic findings include a relatively transverse fracture, low incidence of fracture displacement, and abundant callus (Fig. 31-6). Significant cortical thickening is seen both distal and proximal to the fracture site, and a less dense line is seen at the location of the initial fracture. These findings are often apparent at 12 to 16 weeks.17 Additional diagnostic studies, such as a technetium bone scan or MRI, are rarely needed (Fig. 31-7). There are many theories concerning the cause of these fractures. Richmond and Shafar106 theorized that significant eversion of the hindfoot causing subfibular abutment opposes the pull of the tibiofibular interosseous ligament. The medial pull on the interosseous ligament plus the lateral force on the distal portion of the fibula causes significant stress concentration on the fibula just below the interosseus ligament. In a theory proposed by Devas,29 the potential mechanism for development of the fracture involves the repetitive strong contraction of the plantar flexors of the ankle and the toe flexors from their origin on the fibula with running. This causes a to-and-fro movement of the fibula, ultimately resulting in failure with significant repetitive activity. Stress fractures of the distal fibula are most commonly treated conservatively. Rest and physical activity restriction is the hallmark of treatment. The use of a pneumatic leg brace has been described as giving excellent results, including healing within 6 weeks and resumption of activity.84 Devas29 reported on 50 athletes with fibular stress fractures. Forty fractures occurred within the lateral malleolus. These fractures were treated with elastic strapping and normal weight bearing but no athletic activity until bone tenderness was no longer noted on repeat clinical examination. The typical time to resumption of activity was 6 to 8 weeks. The authors did observe an increased incidence of these fractures with running on hard surfaces, especially in the winter. Thus further recommendations can be made on training conditions and climate. Little has been written of the sesamoids, let alone a stress fracture of these seemingly insignificant bones.4,5 The sesamoids have specific morphologic characteristics that can predispose them to a number of disorders, one being stress fracture.22,77,122 Other than for congenital variations, there are two hallux sesamoids. The medial, or tibial, hallux sesamoid is larger, longer, and often more distally positioned. The lateral, or fibular, sesamoid, is typically smaller, rounder, and more proximal in its relation to the tibial sesamoid. As a result of these differences, the tibial sesamoid endures greater weight-bearing forces and is therefore the one more often injured.4,5,77 The blood supply to the tibial hallux sesamoid is via terminal branches of the medial plantar artery. Ossification occurs at between 9 and 11 years of age, often in multiple areas of the sesamoid. Congenital partition of the tibial sesamoid is fairly common, reportedly ranging from 10% to 33%, ten times greater than that in the fibular sesamoid. Partition is often bilateral, with a wide range of 25% to 85% reported (see Chapter 10).109 The sesamoids function to dissipate forces within the hallux metatarsophalangeal (MTP) joint, elevate the metatarsal head (thereby increasing the mechanical advantage of the flexor hallucis brevis [FHB] tendon), and protect the flexor hallucis longis (FHL) tendon while maintaining its direction of pull. Quite simply, the sesamoids assist with push-off of the hallux. It has been shown that excision of the tibial sesamoid equates with a 10% loss of hallux push-off power.6,7 In addition, removal of the sesamoid can disrupt the delicate tendon balance of the hallux MTP joint, possibly resulting in malalignment. Specifically, a tibial hallux sesamoidectomy can create a disruption of the medial head of the FHB tendon, and a progressive hallux valgus can occur. For these reasons, the sesamoids should not be considered dispensable.77,122 Radiographic analysis includes standing anteroposterior (AP) and lateral foot views. Axial and tangential sesamoid views assist in assessing for focal arthrosis, plantar osteophytes, or bony prominences (Fig. 31-8). An oblique sesamoid view is helpful in identifying a fracture of the tibial sesamoid. It is recommended that a marker (a radiopaque BB) be placed on the skin overlying the site of tenderness to differentiate which sesamoid is indeed involved as well as confirming that it is not the flexor tendon that is the site of pain (see Chapter 10). It is often difficult to differentiate between a fractured and bipartite sesamoid. In general, a fracture exhibits sharp irregular borders on both sides of separation. In contrast, a bipartite sesamoid has smooth cortical edges and a total size larger than that of a single sesamoid. In addition, bipartite sesamoids are often bilateral, and a radiograph of the contralateral foot can assist in the diagnosis.4 Other imaging studies are useful in evaluating sesamoid stress fractures. An MRI is an excellent tool to localize disease and differentiate between bone and soft tissue abnormalities. It can assess sesamoid viability, joint degeneration, and tendon continuity. A bone scan can have a high rate of false positives. A three-phase technique is helpful, with pinhole images to differentiate between the sesamoids. A CT scan can help to identify a stress fracture as well as delineating the degree of metatarsal–sesamoid arthrosis and fracture healing (Fig. 31-9). Figure 31-9 Computed tomography image of the same patient in Figure 31-7 confirming a nondisplaced fracture. Nonoperative treatment may be successful in alleviating the symptoms associated with a sesamoid stress fracture.22 As with nearly all overuse injuries, treatment begins with rest, ice, compression, and elevation. Modification of daily activity and the patient’s training regimen is usually necessary. In an effort to avoid tibial hallux sesamoidectomies, primarily in the athlete with stress fracture nonunions, a bone grafting procedure was described by Anderson and McBryde.5,77 Subsequent studies have shown favorable results with this technique for chronic stress fracture nonunions of the tibial sesamoid in which there was less than 2 mm of diastasis and no gross motion between the two fragments.5 Other contraindications include cystic fragmentation of the sesamoid, avascular necrosis, disproportionately sized fragments, and chondromalacia or frank arthritis of the sesamoid–metatarsal articulation. 1. A general or regional anesthetic is used. 2. Under tourniquet control, a medial–plantar medial skin incision is fashioned over the hallux metatarsophalangeal joint, extending approximately 5 cm (Figs. 31-10 and 31-11). The plantar medial digital nerve should be meticulously dissected and then protected throughout the procedure. Figure 31-10 A medial incision along the hallux metatarsophalangeal joint is used for the bone grafting procedure. 3. A longitudinal capsulotomy is made along the superior border of the abductor hallucis tendon, allowing inspection of the joint and, in particular, the tibial hallux sesamoid (Fig. 31-12). Should the sesamoid have an intact and disease-free articular surface, bone grafting is pursued. Figure 31-12 The capsule and abductor tendon have been longitudinally divided to allow intraarticular exposure of the sesamoid. 4. An extraarticular approach to the sesamoid is made along the plantar aspect of the joint complex, reflecting the overlying FHB tendon. A transverse fissure is identified in the waist of the sesamoid, and the fibrous union is curetted back to viable bone edges (Fig. 31-13). It is very important not to violate the articular surface or create instability between the two fragments. 5. A cortical window is made in the medial aspect of the metatarsal head (Fig. 31-14). Cancellous bone is harvested and packed into the defect created between the proximal and distal poles of the sesamoid (Fig. 31-15). Figure 31-14 A window is created in the medial aspect of the metatarsal head to harvest cancellous bone for grafting. 6. The overlying FHB and soft tissue is reapproximated with absorbable suture. Internal fixation has not been found necessary as long as there is no gross motion between the two fragments. If desired, cerclage suture can be placed around the sesamoid, but axial fixation should be avoided because it can comminute the fragments or lessen the surface area for osseous bridging. Serial radiographs are assessed for union. A CT scan can assist with this determination (Fig. 31-16). Once healed and asymptomatic, typically at 3 months after surgery, running may commence but with the addition of a turf toe insert to minimize hallux metatarsophalangeal dorsiflexion.
Stress Fractures of the Foot and Ankle
Overview
Incidence
Clinical Presentation
Imaging
Treatment
Tibial Diaphyseal Stress Fractures
Clinical Features
Diagnosis
Treatment
Medial Malleolar Stress Fractures
Radiographs
Treatment
Fibular Stress Fractures
Clinical Presentation
Radiographic Findings
Etiology
Treatment
Sesamoid Stress Fractures
Anatomy and Physiology
Diagnosis
Treatment
Conservative Treatment
Surgical Treatment
Sesamoid Bone Grafting
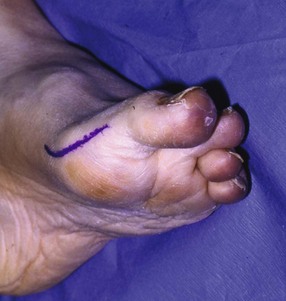
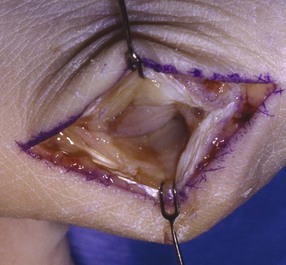
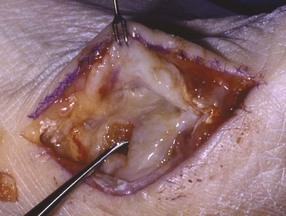
Surgical Technique
Postoperative Care
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree