4.2 Storage and Emptying Disorders of the Bladder
 Anatomy and Physiology of the Lower Urinary Tract
Anatomy and Physiology of the Lower Urinary Tract
A precise knowledge of the anatomic and physiologic relationships thoroughly described in section 1.1 (pp. 1–21) is essential for the physiotherapeutic treatment of the various disorders of the lower urinary tract.
A prerequisite for the assessment and treatment of vesicourethral malfunctions is an understanding of the successive events that take lace during the storage and voiding phases in the bladder, the coordination between the various structures that creates continence, and the neurophysiological mechanisms involved. Because of its importance, a brief overview of the urethral musculature is provided here again.
 Urethral Musculature
Urethral Musculature
Contrary to the earlier view that the smooth-muscle internal sphincter was distinct from a striated external sphincter, the two muscle groups are today considered to be inseparably connected [Oelrich 1983, Dorschner et al. 2001].
The smooth muscle of the internal urethral sphincter in the proximal third of the urethra stretches along the bladder neck, but is not connected to it. Ninety-five percent of the sphincter consists of slow-twitch fibers [Gosling et al. 1981]. There are two layers of smooth muscle in the internal sphincter. The circular muscle layer is poorly developed, while the longitudinal layer is more substantial. The function of the longitudinal muscles is probably to shorten the urethra and close the ureteral ostia of the bladder trigone to prevent urine backing up into the kidneys during micturition [DeLancey 1994].
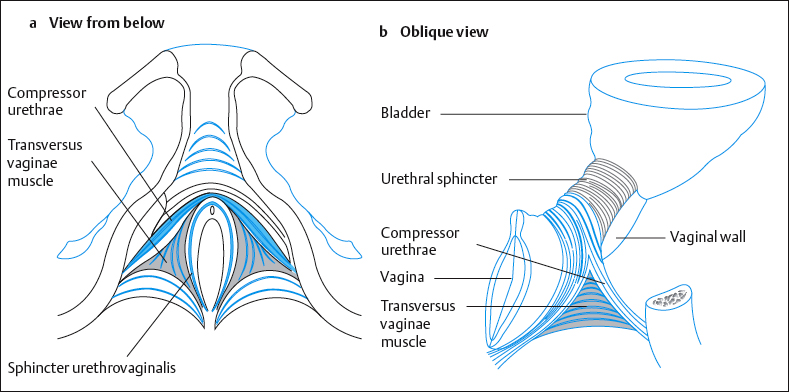
The striated muscle of the urethral sphincter closes the bladder externally. Oelrich [1983] divided it into three segments (Fig. 4.26):
- The striated urethral sphincter loops anteriorly around the middle third of the urethra like a horseshoe. This muscle is also known as the intrinsic rhabdosphincter [Gosling 1981]. It consists primarily of slow-twitch fibers and is therefore especially suited to passive continence [Gosling 1979].
- The compressor urethrae muscle is part of the external urethral complex [Fritsch et al. 2004]. It originates from the rami of the ischium and pubis and runs in a fanlike fashion forward and medially to arch over the anterior part of the urethral surface.
- The sphincter urethrovaginalis is part of the puborectalis, originates from the posterior part of the urethra, and merges into the compressor urethrae lying over it. It runs posteriorly along the urethra and the vagina to merge behind the vagina into the contralateral muscle and the perineum [Fritsch et al. 2004].
- The compressor urethrae muscle is part of the external urethral complex [Fritsch et al. 2004]. It originates from the rami of the ischium and pubis and runs in a fanlike fashion forward and medially to arch over the anterior part of the urethral surface.
All three muscles consist of one-third fast fibers and two-thirds slow fibers. They shorten and lengthen the urethra. The resting tone of these muscles ensures continence even during sleep.
The lower two muscles are thought to be partly responsible for conscious interruption of the urinary stream, although this should never be practiced.
It is interesting to note that nulliparous women take about 2 seconds on average to interrupt the urinary flow midstream, while multiparous women require 4.4 seconds [Sampselle and DeLancey 1992].
Storage Phase of the Bladder
The bladder fills with urine from the kidneys with a negligible increase in pressure (< 10 cm H2O). During this phase, the urethra remains closed and the intraurethral pressure is higher than the intravesical pressure.
In urodynamic studies, the urethral closure pressure is measured as the difference between the intravesical and the intraurethral pressure at the level of the rhabdosphincter. The resting urethral pressure is produced by the tone of the smooth and striated muscle of the urethra, the striated muscle of the pelvic floor, the turgidity of the paraurethral venous plexus, and the structure of the urethral epithelium [Asmussen and Ulmsten 1983]. The urethral closure pressure increases during elevations in intra-abdominal pressure (e. g., laughing, sneezing) and during contractions of the pelvic floor (active transmission). The latter raises the bladder neck into the abdominal pressure zone. The closure pressure can also be raised by transmission of the intra-abdominal pressure on the urethra (passive pressure transmission).
When the bladder is filled to 75%—i.e., 350–500 mL of urine, the intravesical sensory stretch receptors are activated, and the reflex arc mediated by S2–S4 stimulates the bladder muscles to contract. This leads to a strong urge to urinate.
The first urge occurs at about 40 %—i. e., 200 mL—and this can normally be inhibited, allowing the bladder to continue storing urine to a normal full volume [Wyndaele 1990]. The normal upper limit of bladder capacity is usually quoted as 600 mL. The normal tone of the pubococcygeus muscle inhibits the micturition center and prevents activation of the detrusor muscle of the bladder. The storage phase of the bladder is secured [Mahony et al. 1977].
Prerequisites for a Normal Storage Phase
- Good distensibility of the bladder
- Stable detrusor muscle without premature contractions (adequate bladder sensation— i. e., the structures of the central and peripheral nervous system are intact)
- No obstruction between kidney and bladder
- Positive urethral closure at rest and under load.
 Emptying Phase of Bladder
Emptying Phase of Bladder
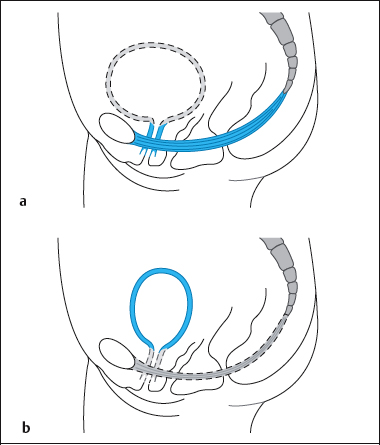
Emptying is triggered by consciously controlled relaxation of the striated pelvic floor musculature and then continues reflexly. The detailed sequence of the process of micturition develops as follows (Fig. 4.27):
- The woman sits or squats.
- The pelvic floor muscles and the periurethral sphincters relax.
- The bladder neck descends.
- The pulmonary diaphragm and the abdominal wall contract.
- The bladder trigone opens the posterior part of the bladder neck by contraction of the longitudinal fibers of the bladder detrusor and by the same action closes the ureteral ostia. The posterior urethrovesical angle flattens.
- The bladder neck forms a funnel into which urine flows (vesicalization).
- The detrusor contracts and the urethra opens, becoming shorter and dilating. Intravesical pressure increases. The posterior angle between bladder and urethra is raised.
- Urine flows until the bladder is empty. The normal urinary flow rate (Q.) is 20 mL/s.
- Next, the bladder muscle relaxes and the urethral closure pressure is restored by the action of the periurethral sphincters and the pelvic floor.
- The bladder trigone resumes its normal tone and the normal posterior vesical angle is restored.
- Next, the bladder muscle relaxes and the urethral closure pressure is restored by the action of the periurethral sphincters and the pelvic floor.
Prerequisites for a Normal Emptying Phase
- Intact neural control
- Adequate functioning of the detrusor
- No increase in resistance to voiding by obstruction
- Adequate pelvic floor relaxation.
 Innervation of the Lower Urinary Tract
Innervation of the Lower Urinary Tract
Good bladder control depends on smooth coordination between the following components of the nervous system:
- Autonomic nervous system
- Sensory system
- Somatic system Central nervous system
- Limbic system
- Skeletal muscle system
- Psyche
Role of the Autonomic Nervous System
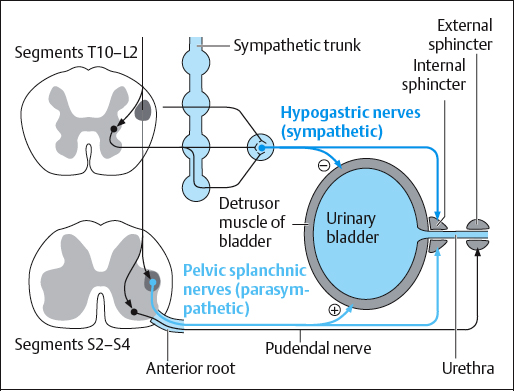
Bladder function is part of the vegetative body functions controlled by the autonomic (sympathetic and parasympathetic) nervous system. The efferent sympathetic fibers leave the spinal cord at T10-L2. From there, they run to synapses in the paravertebral ganglia, then through the superior hypogastric plexus to the bladder. The sympathetic nerves inhibit the bladder muscle and promote continence.
The parasympathetic nerves leave the cord at S2-S4 and reach the bladder through the inferior hypogastric plexus. Their function is to relax the bladder during emptying. The bladder contains receptors arranged in different ways, and these receptors have a number of effects on bladder function.
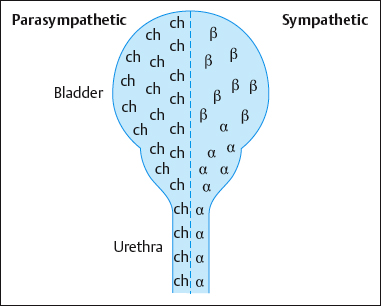
Distribution of receptors in the bladder (Fig. 4.28):
- Release of the postganglionic parasympathetic transmitter acetylcholine stimulates muscarinic receptors in the fundus, causing the bladder to contract.
- The postganglionic sympathetic transmitter norepinephrine has two tasks:
- – It stimulates β-adrenergic receptors in the bladder neck, inhibiting detrusor activity.
- – It stimulates α-adrenergic receptors in the bladder neck, leading to contraction of the internal urethral sphincter.
- – It stimulates β-adrenergic receptors in the bladder neck, inhibiting detrusor activity.
- Release of the postganglionic sympathetic transmitter epinephrine stimulates the βadrenergic receptors to relax the detrusor.
Sensory Control of Bladder Function
The bladder wall contains afferent sensory nerve endings that conduct information concerning the degree of filling, pain, and temperature to the thoracolumbar part of the spinal cord (T10–L2) by way of the hypogastric nerve.
By contrast, the bladder floor and the smooth muscle of the detrusor are supplied by the parasympathetic branch, leaving the sacral part of the cord via the pelvic splanchnic nerves (S2–S4).
The urethra is supplied similarly. Impulses from the sensory nerves of the pelvic floor, the genital skin, the urethral mucosa, and the anal canal are transmitted by the pudendal nerve to the sacral micturition center.
Somatic Control of Bladder Function
The pudendal nerve controls the muscles of the pelvic floor and the striated urethral sphincter. It is a somatic part of the sacral plexus that leaves the spinal cord through sacral segments S2–S4. The pelvic floor relaxes during voiding and ensures continence in the storage phase, especially at night. Through the communication of the pudendal nerve with the parasympathetic splanchnic nerve, the bladder detrusor can be inhibited by voluntary contraction of the muscles of the pelvic floor and the urethral sphincter.
Ultrasound studies of the perineum have shown that a maximum voluntary contraction of the pelvic floor can raise the bladder neck by 8.5 mm [Wise et al. 1993].
Coordinating Centers
The important coordinating centers for micturition lie in the brainstem (reticular formation in the pons), the cerebellum, the limbic system, the hypothalamus, and in the cortex (Table 4.1). The urethrovesical reflex arc through the sacral micturition center (S2–S4) can simultaneously regulate the tone of the muscles of the bladder with the pelvic floor and the urethral sphincters or vice versa.
Storage and voiding of the bladder are regulated by numerous reflexes, which affect the accumulation of urine, retention of urine, release of micturition, maintenance of detrusor contraction, relaxation of the sphincters to ensure complete emptying, interruption of micturition and resumption of the storage phase.
| Micturition center | Function |
|---|---|
| Cortical micturition center (hypothalamus, limbic system, cortex) | Coordination of voluntary control of the micturition reflex, motivation and memory |
| Pontine micturition center (reticular formation) | Coordination of micturition |
| Sacral micturition center (S2–S4) | Involuntary regulation and coordination of pelvic floor muscles, including the striated urethral muscles and the bladder musculature |
By releasing dopamine to the pontine micturition center, the cortical micturition center inhibits premature emptying. Cerebral disease, dementia, or psychological disorders render regulation by dopamine inadequate. Lack of dopamine depresses the inhibition of the vesical sphincter and activates the detrusor.
 Prerequisites for Bladder Control (Continence)
Prerequisites for Bladder Control (Continence)
- A stable detrusor that allows the bladder to fill to an average volume of 300–400 mL without a sharp or continuous rise in pressure (not over 10cm H2O) during filling.
- An adequate, stable urethral closure pressure that is higher than the pressure in the urine-filled bladder and that does not fluctuate spontaneously or with provocation.
- Under physical stress, the increased intravesical pressure must be transmitted passively to the urethra to ensure continence.
- During acute increases in pressure—e. g., coughing—the striated sphincter and pelvic floor musculature must ensure reflex closure of the urethra by actively transmitting the pressure.
- Anatomic structures in the urethra must be intact, including smooth and striated muscle, collagen, and elastic connective-tissue fibers and a mucosa with a vascular submucosa, all of which prevent urine leakage during the storage phase.
- Intact neurological control of the storage and emptying phase.
 Disorders of Storage and Voiding
Disorders of Storage and Voiding
The common bladder disorders (disorders of micturition, incontinence) shown in Table 4.2 are divided into functional deficits of the storage and voiding phases, which may overlap (mixed forms). The definitions used here are based on a publication by the International Continence Society (ICS) [Abrams et al. 1988].
 Causes of Disorders of Micturition
Causes of Disorders of Micturition
Disorders of micturition are symptoms of disorders of bladder storage or voiding of multiple etiologies (Table 4.3).
| Storage disorders | Voiding disorders |
|---|---|
| Stress urinary incontinence | Chronic retention of urine |
| Urge urinary incontinence | Dysuria (painful micturition) |
| Reflex incontinence | Interrupted stream |
| Nocturia (nighttime voiding) | Urinary retention |
| Nocturnal enuresis (bedwetting at night) | Postmicturition dribbling |
| Increased micturition frequency | Repeated micturition |
| Urgency | Hesitancy (difficulty initiating micturition, resulting in a delay in the onset of voiding) |
| Overactive bladder | Diminished urinary stream |
| Extraurethral incontinence | Straining (muscular effort used to initiate, maintain, improve urine stream) |
| Neurogenic detrusor overactivity | Residual urine formation |
| Idiopathic detrusor instability | Detrusor-sphincter dyssynergia |
| Unstable urethral closure | Relaxed bladder (detrusor areflexia, detrusor hypotonia, detrusor acontractility) |
| Increased daytime frequency (pollakisuria) | Overactive urethral closure |
| Micturition disorder | Possible causes |
|---|---|
| Increased frequency, urgency | Urge incontinence, small bladder, large fluid intake, alcohol, medications, bad habits, anxiety, caffeine, nicotine |
| Nocturia (nighttime voiding) | Small bladder capacity, late fluid intake, detrusor overactivity, increased nighttime diuresis |
| Interrupted stream, diminished urinary stream, hesitancy, postmicturition dribbling, repeated voiding | Detrusor hyporeflexia, defective sphincter relaxation, outlet blockage, cystocele, defective urethral closure |
| Straining during voiding, triggering, pressing on the bladder, voluntary retention, residual urine formation | Detrusor areflexia, outlet blockage, detrusor–sphincter dyssynergia (DSD), cystocele |
| Dysuria (painful micturition) | Inflammations, calculi, injuries, sexual abuse, rape |
| Anuria (no voiding in 24 h) | Relaxed bladder, overactive urethral closure, disorders of the upper urinary tract |
Urgency
 ICS definition: The complaint of a sudden, compelling desire to pass urine that is difficult to defer (for fear of leakage).
ICS definition: The complaint of a sudden, compelling desire to pass urine that is difficult to defer (for fear of leakage).
Frequency
 ICS definition: Usually accompanies urgency with or without urge incontinence and is the complaint of the patient who considers that he or she voids too often by day [Abrams et al. 2002].
ICS definition: Usually accompanies urgency with or without urge incontinence and is the complaint of the patient who considers that he or she voids too often by day [Abrams et al. 2002].
 Types of Incontinence and their Etiology
Types of Incontinence and their Etiology
An infrequent urinary dribble is not necessarily a social or hygienic problem. Urinary leakage can be measured by the standardized pad test based on ICS criteria. A urinary loss of more than 2 g is defined as incontinence. It can be graded (Table 4.4).
Table 4.5 presents an overview of the commonest forms of incontinence and their possible causes.
The ICS definitions [Abrams et al. 2002; ICS Fact Sheet 3, 2005] of incontinence disorders have become established in medical terminology.
Stress Urinary Incontinence
 ICS definition: Involuntary leakage of urine on effort or exertion, or on sneezing or coughing. Some studies define stress urinary incontinence as any loss of urine, while others define it as loss of urine more often than twice a month [Bumpand Norton 1998].
ICS definition: Involuntary leakage of urine on effort or exertion, or on sneezing or coughing. Some studies define stress urinary incontinence as any loss of urine, while others define it as loss of urine more often than twice a month [Bumpand Norton 1998].
Urge Urinary Incontinence
 ICS definition: Urge urinary incontinence is the complaint of involuntary leakage accompanied by or immediately preceded by a strong desire to void [ICS Fact sheet 3, 2005; Abrams et al. 2002].
ICS definition: Urge urinary incontinence is the complaint of involuntary leakage accompanied by or immediately preceded by a strong desire to void [ICS Fact sheet 3, 2005; Abrams et al. 2002].
There are two types: sensory and motor urge urinary incontinence.
Overactive Bladder (OAB)
 ICS definition: OAB is a symptom syndrome that is defined as urgency, with or without urge incontinence, usually with frequency and nocturia (sleep disturbing voiding) [Abrams et al. 2002].
ICS definition: OAB is a symptom syndrome that is defined as urgency, with or without urge incontinence, usually with frequency and nocturia (sleep disturbing voiding) [Abrams et al. 2002].
 Mixed incontinence: The simultaneousoccurrence of stress and urge urinary incontinence.
Mixed incontinence: The simultaneousoccurrence of stress and urge urinary incontinence.
Nocturnal Enuresis
 ICS definition: The complaint of loss of urine occuring during sleep [Abrams et al. 2002].
ICS definition: The complaint of loss of urine occuring during sleep [Abrams et al. 2002].
Nocturia
 ICS definition: Complaint that the individual has to wake at night one or more times to void.
ICS definition: Complaint that the individual has to wake at night one or more times to void.
Chronic Retention of Urine
 ICS definition: A nonpainful bladder which remains palpable or percussable after the patient has passed urine. Such patients may be incontinent [Abrams et al. 2002].
ICS definition: A nonpainful bladder which remains palpable or percussable after the patient has passed urine. Such patients may be incontinent [Abrams et al. 2002].
This may be, but is not necessarily, connected with a detrusor contraction. Chronic retention of urine can develop as a result of obstruction (obstructive overflow incontinence, OOI), defective or absent detrusor contraction or overdistension of the bladder (nonobstructive overflow incontinence, NOI). The major problem resulting from this type of incontinence is the danger of urine reflux or renal back-pressure.
Neurogenic Detrusor Overactivity
 ICS definition: Loss of urine due to neurogenic detrusor overactivity and/or involuntary urethral relaxation in the absence of the sensation, usually associated with the desire to micturate [Abrams et a 1988].
ICS definition: Loss of urine due to neurogenic detrusor overactivity and/or involuntary urethral relaxation in the absence of the sensation, usually associated with the desire to micturate [Abrams et a 1988].
| Grade | Loss of urine |
|---|---|
| 1 | When coughing, sneezing, laughing, straining |
| 2 | When walking, carrying, lifting |
| 3 | While lying down |
The exact definitions depend on the level of the lesion.
Lesion of peripheral nerves. Damage to the pelvic plexus can result from surgery or injuries in the true pelvis. It can lead to neurogenic detrusor overactivity with increased urinary frequency. The detrusor–sphincter synergy remains intact.
Damage to the pudendal nerve leads to impaired innervation to the muscles of the pelvic floor and hence to insufficient urethral closure pressure.
Spinal reflex incontinence (SRI). If the ascending and descending tracts are completely interrupted, the urge to void cannot be felt and voluntary control of the detrusor is lost. The sacral reflex accomplishes voiding.
When the detrusor and sphincter are not coordinated, the result is a detrusor–sphincter dyssynergia (DSD). Voiding is incomplete, resulting in overflow/dribbling, hesitancy, weak stream, a feeling of incomplete emptying, and residual urine. Involuntary loss of urine may occur. Detrusor–sphincter dyssynergia occurs primarily in neurological disorders—e. g., multiple sclerosis.
Motor paralytic bladder. If an incomplete lesion spares primarily afferent tracts, the desire to urinate may be maintained, but voluntary control is lost. The result is detrusor–sphincter dyssynergia with dribbling and residual urine. Involuntary loss of urine may occur.
Supraspinal reflex incontinence (SSRI) (also known as uninhibited neurogenic bladder). The absence of cortical inhibition is marked by urgency, increased frequency of micturition and incontinence. The bladder can be emptied completely, but it is often too late. In this lesion, the synergy between detrusor and sphincter is intact.
| Type of incontinence | Possible causes |
|---|---|
| Stress urinary incontinence | Incompetent urethral closure mechanism due to:
|
| Urge urinary incontinence | Motor:
|
| Nocturia |
|
| Chronic retention of urine |
|
| Extraurethral incontinence |
|
| Neurogenic detrusor overactivity |
|
Extraurethral Incontinence (EUI)
The bladder does not empty through the urethra. This may be due to urogenital fistulas or an ectopic ureter (Table 4.5).
 Prevalence of Incontinence
Prevalence of Incontinence
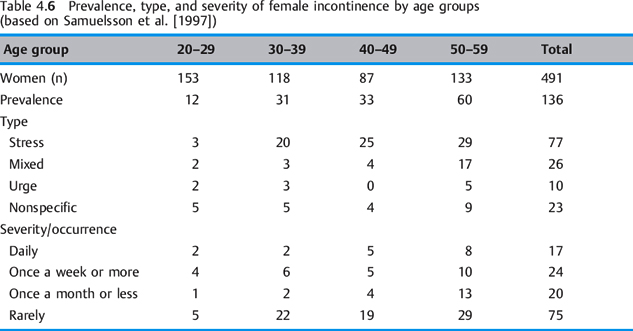
The variation in the reported prevalence of incontinence relates to studies that lack uniformity in their data collection. Criteria such as the frequency of incidents of incontinence (e. g., more than twice a month or a single episode of urine loss in the past 12 months), the age of the women, and the method of questioning (oral or in writing) could influence the number of those affected. In Germany, the official statistic for those affected is 3 million, but the unreported figure for this illness, which is often regarded as a taboo subject, is likely to be twice that number. In the USA, the number of those affected is estimated to be at least 14 million, and its total costs may amount to as much as $46 billion a year [Wagner and Hu 1998]. The prevalence of stress incontinence after vaginal delivery is estimated as 0.7–38.0%, depending on the researcher [Dimpfl et al. 1992, Morkved and B⊘ 1999, Viktrup et al. 1993]. Table 4.6 shows the generalincrease in the prevalence of incontinence as well as the predominance of stress urinary incontinence in all age groups and the increasing severity with a woman’s age.
 Vesical and Urethral Dysfunction
Vesical and Urethral Dysfunction
Incontinence and disorders of micturition are expressions of vesicourethral dysfunctions of various origins. The following set of disorders is classified according to the site of the malfunction.
Vesical Dysfunctions
Neurogenic detrusor overactivity. Phasic: characteristic wave form may or may not lead to UI. Terminal: typically associated with reduced bladder sensation, with no sensation in complete SCI [Abrams et al. 2002].
Detrusor hypocontractility. The detrusor must contract during voiding and maintain this contraction until the bladder is completely empty or until voiding is interrupted voluntarily. If the detrusor contraction is not strong enough, the bladder is not able to empty completely (residual urine, repeated micturition), or emptying may be unacceptably prolonged, or both.
Detrusor acontractility. When the normal bladder capacity of 300–500 mL is reached, the detrusor has to contract, since otherwise voiding cannot be initiated and there is a risk of back-pressure affecting the kidneys.
Urethral Dysfunctions
Unstable urethral closure. Normally, the bladder neck and the urethra have to remain closed during filling of the bladder, even if abdominal pressure rises.
If urethral closure is incompetent, urine escapes even when the intravesical pressure does not rise. The urethral closure pressure is not sufficient to prevent loss of urine, especially when intra-abdominal pressure rises. Clearly, loss of urine is preceded by a brief decline in urethral pressure.
 The definition of this dysfunction is a spontaneous fall in maximal urethral closure pressure to one-third or less [Wise et al. 1993], or below 15 cm H2O[Vereecken et al. 1985].
The definition of this dysfunction is a spontaneous fall in maximal urethral closure pressure to one-third or less [Wise et al. 1993], or below 15 cm H2O[Vereecken et al. 1985].
Hyperactive urethral closure. If the urethra either does not open or does not open completely during voiding, the condition is called hyperactive urethral closure. The consequences are incomplete voiding and frequent micturition.
Detrusor–sphincter dyssynergia. A disturbance in the interaction between detrusor function and urethral function, which are normally antagonistic rather than synergistic, is known as detrusor–sphincter dyssynergia (DSD). This leads to severe voiding problems. The condition is caused by neurologic diseases above the sacral micturition center (e.g., after high spinal cord injury).
| Medication | Effect |
|---|---|
| β-adrenergic antagonists (β-blockers) Prostaglandins | Increased detrusor contractility, increased frequency and urge urinary incontinence |
| Alcohol | Sedation, smooth muscle relaxation, diuresis; makes incontinence worse |
| Caffeine | Increased excretion; increased urgency |
| α-adrenergic antagonists (α-blockers) Muscle relaxants | Reinforce existing stress urinary incontinence (by reducing sphincter tone and bladder neck outlet resistance). They are also used in drug treatment for voiding dysfunctions |
| Diuretics (used to remove fluid) Reinforce incontinence (by increasing urine excretion, polyuria) in diabetes, cardiac failure | Reinforce incontinence (by increasing urine excretion, polyuria) |
| Estrogens | Restore collagen and improve submucosal blood perfusion |
| Tricyclic antidepressants Antihistamines Antiasthmatic agents Antiallergic agents Neuroleptics Hypnotics Antiparkinsonian agents Ophthalmic agents | Reinforce voiding disorders (urine retention by anticholinergic effect, –receptor stimulant action) |
An overactive pelvic floor musculature or an overactive striated urethral sphincter can also cause voiding problems, in the absence of neurological disease.
 The Effect of Medications
The Effect of Medications
The ingestion of various medications and addictive substances influences the storage and voiding phases of the bladder (Table 4.7).
 Testing Procedures and Examinations
Testing Procedures and Examinations
Disorders of micturition and incontinence are symptoms of vesicourethral dysfunction and often accompany various primary diseases. Secondary diagnoses of this type require specialized diagnostic procedures.
A number of different procedures are used to narrow down the patient’s specific problem and provide a basis for physiotherapeutic interventions. Both medical and physiotherapeutic procedures are available for this purpose. Urologists, gynecologists, neurologists, or family physicians may treat the affected patients. Having obtained a medical diagnosis and prescription, the patient may then seek out an outpatient physiotherapy clinic.
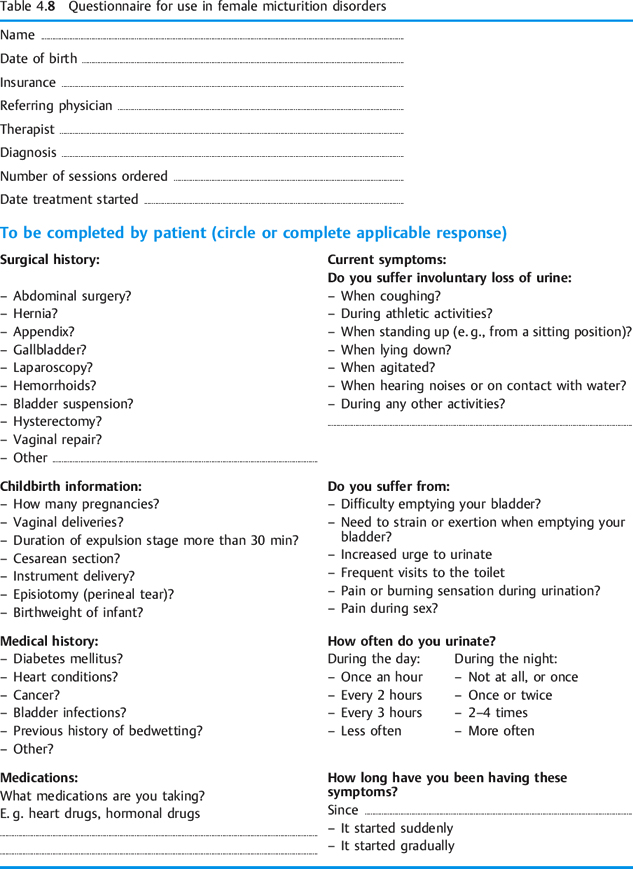
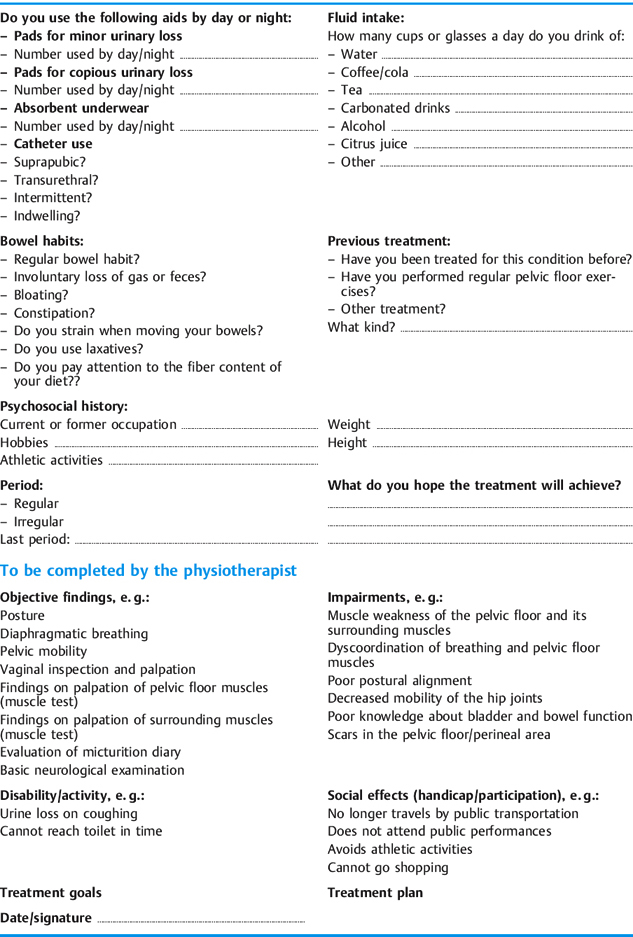
In enumerating the types of examination available, I have confined myself specifically to storage and voiding problems of the bladder. In premenopausal women, the examination and repeat testing should be timed in accordance with the menstrual cycle. Tests conducted in the second half of the cycle often show poorer results. Obviously, other physiotherapeutic examinations need to be included as necessary (posture, other muscle tests, tests for mobility, etc.). A clinical questionnaire for patients with voiding problems is presented in Table 4.8.
Subjective Data
Apart from information about age, occupation, hobbies, and general activities, more targeted questions should address specific problems. Standard questionnaires can be helpful in this respect. In order to plan treatment, the patient’s motivation and therapeutic goal should be made explicit.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree