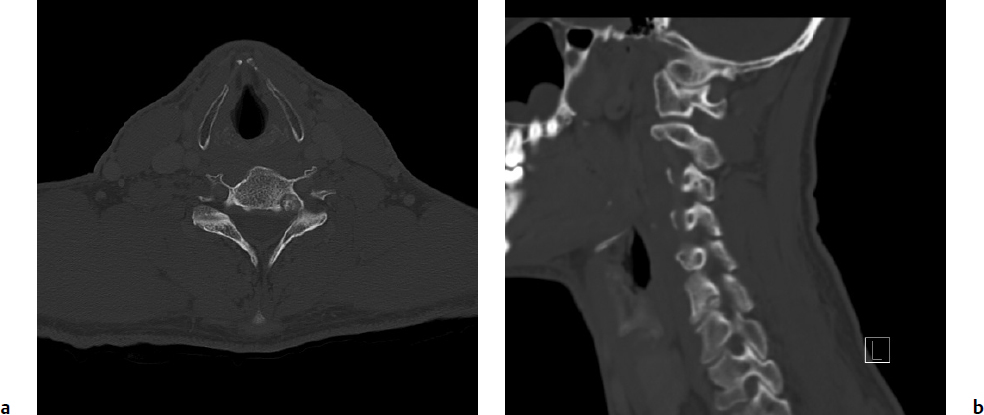
6 Osteoid osteoma and osteoblastoma are solitary lesions that comprise the osteoblastic benign primary tumors of the spine. Despite the fact that they are histologically extremely similar, osteoid osteomas are not known to progress to osteoblastomas. Their distinction is largely based on their respective size and differing biological behavior. Osteoid osteomas are typically less than 2 cm in diameter, whereas osteoblastomas are larger than 2 cm. With regard to their biological behavior, osteoid osteomas run a predictable benign course with an effective response to treatment.1,2 Conversely, osteoblastomas exhibit a spectrum of behaviors from relatively latent to extremely aggressive, with occasional malignant transformation to low-grade osteogenic sarcomas or “malignant osteoblastomas” 12 to 25% of the time.3 Both the likelihood of local recurrence and the treatment of osteoid osteomas and osteoblastomas differ dramatically. Osteoblastomas are one of the most challenging benign spine tumors to treat because of a high preponderance for local recurrence, difficulty in predicting their behavior, and the limited treatment options. Osteoid osteomas are four times more common than osteoblastomas overall.4 Osteoblastomas make up 10 to 25% of all primary osseous spine tumors.2 Both types of lesion are seen mainly in the long bones; only 10 to 20% of osteoid osteomas and 40% of osteoblastomas occur in the spine.1,4–6 Osteoid osteomas are more prevalent in the lumbar spine, whereas osteoblastomas do not have a predilection for any particular spinal region. Both lesions are seen more commonly in males than females (2–4.5:1) in their late teens to early 20s.5,7–10 The most common age of presentation is in the second to decade of life. Eighty percent to 100% of patients present with neck or back pain, which is usually localized4; 25 to 62% of patients with an osteoid osteoma or an osteoblastoma have a scoliosis at the time of presentation (more common with osteoid osteoma),7,8,11 and 45% of cervical cases present with torticollis.9 These findings are both the result of an inflammatory effect on paraspinal muscles, causing asymmetric muscle spasms.11 In the immature thoracic and lumbar spine, this leads to growth inhibition of the vertebral epiphysis and a rotational deformity, resulting in rapidly progressive curve at a high risk of becoming structural.12 The vast majority of spinal osteoid osteomas and osteoblastomas affect the posterior elements.4,10,13 Due to their larger size, osteoblastomas may extend into the anterior vertebral body and the spinal canal, thus potentially causing neurologic symptoms.1,2,14 Because of their different behaviors, prognosis, and treatments, it is critical to fully investigate these lesions with the appropriate imaging and biopsy in order to confirm the accurate diagnosis of osteoid osteoma, osteoblastoma, or osteosarcoma. Benign musculoskeletal neoplasms are classified according to the Enneking staging system, which has been shown to be reliable and valid in the spine.15 This system helps the clinician assess tumor behavior and helps guide treatment. Enneking’s surgical principles dictate which treatment is most appropriate for each stage (Fig. 6.1). It is essential for the oncological spine surgeon to completely assimilate the original definitions of the terms as described by Enneking.16,17 Terms used to describe margins include intralesional (some tumor left behind), marginal (dissection along the tumor capsule or reactive zone), or wide (cuff of healthy tissue around the tumor is removed), and they must be distinguished from the different surgical techniques used to achieve these margins. These typically include the piecemeal technique, where the tumor is removed in several different pieces (e.g., curettage), or en bloc, in which the tumor is removed in one piece, regardless of the surgical margins. Another important distinction is that of the surgical and histological margins. The surgical margins describe the surgeon’s planned and gross impression of the extent of resection intraoperatively. In contrast, histological margins are obtained when the intraoperative sample is sent to the pathology lab, and a microscopic analysis serves as the final verdict for surgeons regarding their success at achieving the planned surgical margins (intralesional, marginal, or wide).16,17 Fig. 6.1a,b A 40-year-old man presented with an insidious onset of left-sided neck pain radiating to his left scapular region, slowly progressive over 10 months. His pain was bothersome at night, and relieved with nonsteroidal anti-inflammatories. He denied any neurologic symptoms. Axial (a) and sagittal (b) computed tomography (CT) scans showed a well-circumscribed lesion with mineralization in the nidus, radiolucent rim (representing the portion of the nidus that is not mineralized), and sclerotic rim. It measured 8 mm in diameter. Clinical and radiographic features were consistent with an osteoid osteoma, and the patient was therefore treated with radiofrequency ablation, resulting in a complete resolution of his symptoms. Osteoid osteomas account for approximately 10% of primary bone tumors.18 They are bone-forming tumors with limited growth potential that range from 15 to 20 mm in diameter. Patients typically present with constant or episodic pain that increases at night or with physical activity. The pain is caused by the presence of nerve endings within the tumor, which are stimulated by vascular pressure and the production of prostaglandins.14 Symptoms classically respond to nonsteroidal anti-inflammatory drugs (NSAIDs), at least initially. Osteoid osteoma is the most common cause of painful scoliosis in adolescents.18 On plain radiographs, osteoid osteomas may show an osteosclerotic lesion, with or without a visible radiolucent nidus, but they are often missed because of their location in the posterior elements and relatively small size (< 2 cm). On computed tomography (CT) scan, they are characterized by sclerosis surrounding a radiolucent nidus, and periosteal bone reaction is often seen. A central region of mineralization may be present. Magnetic resonance imaging (MRI) is best used to aid in preoperative planning to determine proximity to neurologic structures.19 On T1- and T2-weighted images, the calcification within the nidus and surrounding sclerosis are both of low signal intensity. Enhancement of the vascular nidus with gadolinium may be seen. If neither of these imaging modalities depict a lesion, the most sensitive tool in the diagnosis of osteoid osteoma would then be a technetium bone scan, which shows intense radionucleotide uptake at the nidus, and a less intense larger area of uptake surrounding it.20,21 If there is any doubt radiographically and clinically that this may be something other than an osteoid osteoma, a biopsy is indicated. This is further discussed in the osteoblastoma section of this chapter. Treatment options for osteoid osteoma consist of conservative treatment with NSAIDs, surgical excision (most commonly intralesional curettage or en-bloc excision), or percutaneous interventions. In adults, conservative management is the first line of treatment; if this fails, or if avoidance of long-term use of anti-inflammatories is preferred, surgical excision is indicated. In younger patients, surgical excision is generally recommended early, to prevent a scoliotic deformity. In cases where spinal deformity is already present, resection of the lesion can result in reversal of this deformity if the lesion is resected within 15 months of its onset.7,18 Conventionally, the surgical excision of osteoid osteoma has been considered acceptable treatment for cure.7,9,22–24 More recently, less invasive techniques, such as (ILP) and percutaneous radiofrequency ablation (RFA), initially employed in the appendicular skeleton, have begun to gain favor for use in the spine. These techniques have several advantages: they can be performed under local anesthesia, therefore simultaneous neurologic examination is possible; they cause minimal surgical trauma, and in turn patients experience less postoperative pain and have a decreased risk of impairment and infection; and they are associated with a shorter recovery period, shorter hospital stay, and thus lower cost.14,25–27 On the other hand, the risk of causing local neural element injury with these techniques has been a significant concern in relation to their use in the spine. Specifically regarding RFA, heating a needle tip to 90°C for 4 to 6 minutes in the nidus of a lesion raises concerns about thermal injury to nearby neural elements. However, an ex-vivo study has shown that there is no temperature increase within the spinal canal when an intact cortex separates neural structures from the needle tip, suggesting an insulating effect of cortical bone.28 Furthermore, several recent studies have demonstrated the efficacy and safety of RFA when used in the spinal column.29,30 Martel et al29 treated 10 patients with 4 to 6 minutes of RFA aimed at the nidus of osteoid osteomas. The distance between the nidus and adjacent neural tissue was 2 to 12 mm (mean, 5 mm). There were no complications reported. Two patients (20%) required a second similar RFA treatment due to recurrence of pain at 2 months. Repeat treatment resulted in long-term symptom resolution. Vanderschueren et al30 treated 24 patients with spinal osteoma with RFA. They reported a success rate of 79% (19/24) after a single treatment, and 96% (27/28) after a second treatment. There were no complications, and all patients were discharged from the hospital on the same day as the procedure. They concluded that CT-guided RFA should be the treatment of choice in lesions located at least 2 mm away from neural tissue, whereas surgical excision is advised for lesions adjacent (< 2 mm) to neural structures. Several other reports of a smaller number of patients with osteoid osteoma in the lamina, transverse process,31 lumbar vertebral body,27,32,33 pedicle,28 and spinous processes34 successfully treated with RFA support this as a treatment option for spinal osteoid osteoma lesions as well. Osteoblastomas are benign bone-forming neoplasms similar to osteoid osteomas. However, they have an unlimited growth potential, being larger than 2 cm by definition, and are clinically more aggressive. Patients with this type of lesion present with dull back pain or with symptoms of neurologic compression. In fact, 25 to 50% of patients present with a neurologic deficit.7,35 In contrast to osteoid osteoma, symptoms typically are not different at night and respond poorly to NSAIDs. Scoliosis is also known to occur in spinal osteoblastoma, but less frequently than with osteoid osteoma. Osteoblastomas can generally be categorized into two types: active and aggressive. The distinction between the two is critical, as it significantly impacts treatment and outcome. Unfortunately, this distinction is not easily made or well established in the literature, and therefore one must use a constellation of clinical and imaging findings. Investigators should use the Enneking staging system (Table 6.1) as a basis to categorize lesions; stage 2 lesions are labeled as active, and stage 3 as aggressive.1,2 Stage 2 lesions have well-defined borders, combined osteolytic and sclerotic features (often resembling osteoid osteoma where a sclerotic ring surrounds a lytic nidus), and no soft tissue involvement. Stage 3 lesions are more rapidly growing, usually exceeding 4 cm in diameter, and have a more destructive appearance on imaging. Their margins are poorly defined, they are primarily lytic, and they erode the cortex to invade the spinal canal and surrounding soft tissues. Some are very expansile, resembling an aneurysmal bony cyst.2 The clinical course of both differ as well, with active lesions causing slowly progressive pain with or without spinal deformity secondary to muscle spasm, and aggressive lesions causing a faster progression of symptoms with possible early neurologic complaints. Another differentiating feature may be found histologically: aggressive osteoblastomas comprise large epithelioid osteoblasts, characterized by abundant eosinophilic cytoplasm twice the size of conventional osteoblasts.36 Table 6.1 Enneking Staging System for Benign Primary Spinal Neoplasms
Spinal Osteoid Osteoma and Osteoblastoma

 Introduction
Introduction
 Staging and Terminology
Staging and Terminology
 Osteoid Osteoma
Osteoid Osteoma
Imaging
Treatment
 Osteoblastoma
Osteoblastoma
| Stage | Description | Margin for Control |
| 1 | Latent: usually asymptomatic | Intracapsular |
| 2 | Active: locally symptomatic | Marginal, or intracapsular + adjuvant therapy |
| 3 | Aggressive: symptomatic with local invasion and/or destruction of tissues with potential for metastasis | Wide, or marginal + adjuvant therapy |
Source: Adapted from Enneking WF. A system of staging musculoskeletal neoplasms. Clin Orthop Relat Res 1986;204:9–24.
Imaging
On plain radiographs, 50% of osteoblastomas are lytic, 30% are sclerotic, and 20% are mixed8; 55% are found entirely in the posterior elements, 42% involve the posterior elements and vertebral body, and 3% involve the vertebral body exclusively.36 Depending on whether they are active or aggressive, they have a different radiological appearance as described in the previous paragraph. Unlike osteoid osteomas, osteoblastomas are typically readily visible on plain radiographs. CT scanning is optimal preoperatively to precisely locate the tumor and define the extent of bony involvement, whereas MRI is helpful in identifying intra- and extraosseous reactive changes, infiltration into surrounding soft tissues or spinal canal, and neurologic compression. The appearance of osteoblastoma on MRI, however, is generally non-specific and may overestimate the size of the lesion due to local inflammation and edema.18
Histology
The differential diagnosis of osteoblastoma includes aneurysmal bone cyst, giant cell tumor, osteosarcoma, Ewing’s sarcoma, cartilaginous tumors (enchondroma, osteochondroma, and chondrosarcoma), and osteomyelitis. Therefore, a biopsy is indicated prior to undertaking treatment.
Histological examination of osteoblastomas is characterized by a fibrovascular stroma and a nidus containing osteoblasts that produce osteoid tissue and woven bone. This is similar in osteoid osteomas, but, in addition, osteoblastomas commonly have large vascular spaces and reactive giant cells.10,13 Aggressive (stage 3) osteoblastomas are more cellular, with swollen, plump osteoblasts,13 and may have a multifoci growth pattern mimicking permeation. These features are also present in osteosarcoma; their foci are microscopically identical to those of aggressive osteoblastomas. However, true permeation of surrounding tissues and lack of “maturation” at the edges of the tumor are characteristic of osteosarcomas and can be used to differentiate osteosarcoma from aggressive osteoblastoma. This can easily be missed, and the review of the histology by an experienced musculoskeletal oncologist is recommended.37
Treatment
Osteoblastomas are managed surgically because of the recalcitrant nature of the pain they cause, as well as due to their locally aggressive behavior; their larger size leads to potentially significant bony destruction, deformity, instability, or neurologic compression.18 Treatment options consist of intralesional curettage with bone grafting or cementation, or en-bloc resection. The former is recommended for active (Enneking 2) osteoblastomas, and the latter for aggressive (Enneking 3) osteoblastomas; the spine’s challenging structural and neurologic anatomy makes it extremely difficult to follow these guidelines.2
En-bloc resection with marginal or wide margins is more invasive and imposes greater morbidity on patients, but is justified by the high recurrence rate and possibility for malignant transformation of aggressive osteoblastomas when treated with a more conservative approach.1,2 Schajowicz and Lemos38 treated a series of aggressive osteoblastomas and recurrence rates were 100% (4/4) for the ones treated with intralesional curettage, 20% (1/5) for the ones treated with excision, and 0% (0/3) for the ones treated en bloc. In a clinically based systematic review done in collaboration with the Spine Oncology Study Group,2 aggressive osteoblastomas were reported to recur at a rate of 50% when treated with subtotal resection, compared with 10 to 15% in less aggressive lesions. Despite weak evidence, the consensus expert opinion recommendation is to treat aggressive lesions with marginal or wide en-bloc excision, and nonaggressive lesions with intralesional curettage.2
Another factor that was found to affect the rate of recurrence is whether or not the lesions had been previously treated with intralesional curettage (and therefore were recurrent lesions) or had undergone an open biopsy. Boriani et al1 showed that patients who had either an open biopsy or curettage had a recurrence rate of 67% (2/3 patients) after en-bloc resection, and 75% (3/4 patients) after a second intralesional curettage. This compares with a recurrence rate of 0% (0/10 patients) after en-bloc resection, and 7.1% (2/30 patients) after a second intralesional curettage in patients whose lesions were intact (no previous procedure at that site). Lucas et al,36 in a review of 306 cases, found that there was recurrence after en-bloc only when the procedure was performed through a prior resection cavity. This highlights the importance of the correct diagnosis initially, including the differentiation of aggressive versus active lesions, and appropriate treatment based on this diagnosis, at the time of initial treatment.
Adjuvant Therapies: Radiation and Chemotherapy
The role of radiation and chemotherapy for incomplete surgical resection of osteoblastoma is not well defined. When aggressive lesions are not completely excisable due to anatomic (e.g., involvement of significant neural elements) or medical considerations, they have a higher likelihood of recurrence. If intralesional resection is the only option, postoperative radiation therapy is the most commonly used adjuvant therapy and is considered reasonable. Radiotherapy is also used in recurrent lesions, most often after repeat intralesional curettage. The benefit of this modality, however, is still very controversial, with the majority of cases showing no advantage and a minority of cases demonstrating some benefit.6,39 Marsh et al6 concluded, based on a review of 197 osteoblastoma cases, that radiation therapy does not alter the course of the disease and appears to be contraindicated. In contrast, Boriani et al1 reviewed a series of 30 osteoblastoma cases and found that recurrences occurred in five of 22 patients treated with intralesional curettage alone, compared with no recurrences occurring in the 15 patients treated with both curettage and radiation (at 2-year follow-up). Harrop et al,2 in their systematic review, concluded that the evidence was again weak, but that radiotherapy in recurrent lesions or incompletely resected aggressive osteoblastomas should be considered as a treatment option.
The use of adjuvant chemotherapy is limited to anecdotal cases, and evidence to support its use is even weaker than for radiotherapy.2 There are a limited number of case reports and anecdotes of recurrent osteoblastomas that underwent reexcision or radiation.40–42 In all cases no further recurrence occurred when the chemotherapy was utilized; however, this success was often attributable to the surgical management only, with no demonstrated additional benefit of chemotherapy. Harrop et al2 concluded in their systematic review that there is a limited role for chemotherapy in recurrent aggressive osteoblastoma. Further research is required to clarify the role of both these adjuvant treatments in the management of osteoblastoma.
 Clinical Cases
Clinical Cases
Pertinent clinical cases are presented in Figs. 6.1, 6.2, 6.3, 6.4.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree