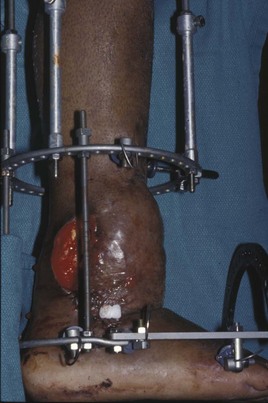
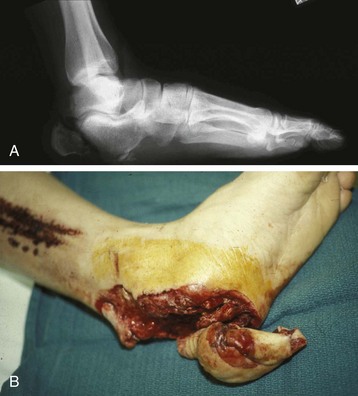
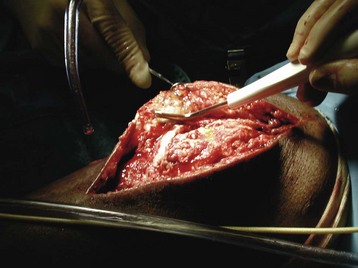
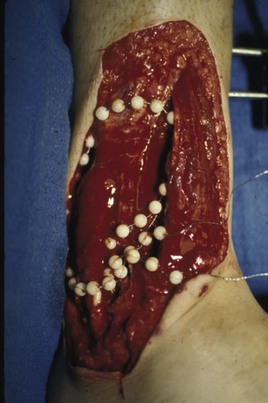
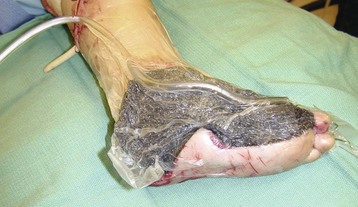
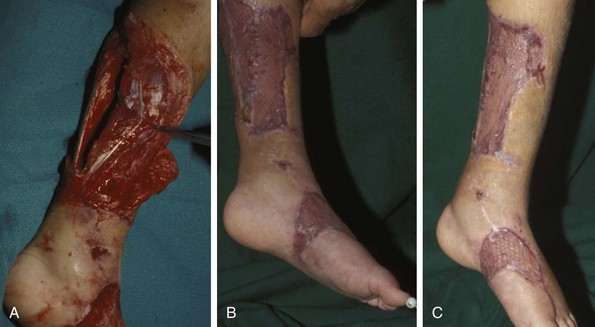
Chapter 17 COMPLEX MUSCULOSKELETAL INJURIES SELECTION OF RECONSTRUCTION TIME PREPARATION OF THE WOUND FOR RECONSTRUCTION TIMING OF FREE TISSUE TRANSFER SELECTION OF TISSUE FOR TRANSPLANTATION INFORMED CONSENT AND MEDICAL AND LEGAL ISSUES TEAM APPROACH TO EXTREMITY RECONSTRUCTION RECONSTRUCTIVE ALGORITHM BY REGION Group A: Closed Fractures Treated by Open Reduction and Internal Fixation Group B: Requirements for Free Flaps for Foot Reconstruction TRAUMA AND THE DYSVASCULAR AND DIABETIC FOOT AESTHETIC CONSIDERATIONS AFTER LIMB TRAUMA Open fractures have plagued surgeons since the time of Hippocrates; drawings from his original writings show crude attempts at external fixation for the purpose of examining and treating wounds.2 Incomplete documents from the ancient Egyptian period reported that comminuted fractures were treated expectantly. “Compound fractures” were considered a fatal injury because amputation was not a part of the surgical arsenal.3 In the sixteenth century, Ambroise Paré, one of the founding fathers of orthopaedics, warned against the potentially life-threatening condition of gangrene resulting from open fractures; he revolutionized amputation surgery by use of the tourniquet and introduced the hemostatic clamp and vascular ligatures.68 Important advances have taken place in the field of soft tissue reconstructive surgery, including the introduction of local flaps and free flaps as efficient methods of closing large posttraumatic soft tissue defects. A better understanding of the anatomy and physiology of free flaps, with resultant high rates of success using autologous tissue transfers, and the introduction of distally based fasciocutaneous flaps have improved the surgeon’s ability to close soft tissue defects of the foot and ankle.57 Initial treatment of fractures has improved significantly with the development of antibiotic therapy and aseptic surgical procedures in conjunction with improved stabilization techniques. This progress has increased limb salvage in the treatment of severe injuries to the foot and ankle at risk for amputation. Today’s acute fracture management with early antibiotic treatment, irrigation and debridement, and acute fracture stabilization has led to a significant reduction in fracture infection rates (Fig. 17-1). The basic principles of open reduction and internal fixation, defined by the Arbeitgemeinschaft für Osteosynthesesfragen (AO) group five decades ago,93 included anatomic reduction and stable internal fixation, careful attention to soft tissue handling, and functional rehabilitation of the injured limb—all vital to fracture management. Functional rehabilitation involves restoring muscular power and normal biomechanics. The importance of soft tissue reconstruction has been emphasized over the last two decades. New fixation techniques, such as indirect reduction and biologic plating with implants that respect biology and avoid compromised periosteum around bone, are now being used. Compatible implants, such as the locking plates and calcaneal plates with improved metallurgy, were designed to limit the damage to soft tissue caused by overvigorous dissection and stripping of the soft tissue envelope.85 The surrounding soft tissue has been recognized as the vascular envelope responsible for nurturing bone back to health. The importance of its reconstruction early in the posttraumatic course cannot be overemphasized; neither can the importance of surgical anatomy, internervous planes, vascular territories, and atraumatic techniques of dissection.69,115 Rather than dissection techniques that result in devascularization of bone and soft tissue, a keener awareness of delicate soft tissue handling and atraumatic technique by the orthopaedist contributes to the prevention of adverse iatrogenic sequelae after injury. Proper handling of soft tissue includes tools such as skin hooks that permit manipulation of skin and tissue flaps without further damage to the soft tissues. Muscles are richly vascularized structures that power the locomotor system. They have one of five blood supplies, as outlined by Mathes and Nahai.84 Muscles can be manipulated as transposition flaps, island pedicle flaps, and free tissue transplantations (free flaps). All of the layers of soft tissue just described, including the periosteum, will accept a split-thickness skin graft (Fig. 17-2). Despite contour irregularity, if these layers are healthy and well vascularized, a skin graft can be applied to seal the open wound. This is the first goal in wound management, that is, to reconstitute the epithelial surface of the extremity. Figure 17-2 A meshed split-thickness skin graft applied over a free latissimus myocutaneous transfer. A logical method of reconstruction of the soft tissues must be developed to allow bone to heal and limbs to function normally. The algorithm should be capable of being used by the foot surgeon in the setting of acute or chronic soft tissue injury with or without fractures. In addition, it should be applicable to chronic conditions, such as osteomyelitis, nonunion, or tumor.26 Assessment of soft tissue injury is necessary in both open and closed fractures. The degree of soft tissue injury provides a prognosis and guides fracture management. The different classification schemes can be fairly simple or minutely detailed. The simpler schemes are noncomprehensive and inexact but are the most likely to be used.46 As the significance of soft tissue injuries on the influence of bone healing became more apparent, Gustilo and Anderson43 devised a three-grade classification in 1976. Type 1 fractures have a clean wound smaller than 1 cm, type 2 wounds have a laceration greater than 1 cm and without extensive soft tissue damage, and type 3 wounds are severe soft tissue lacerations with segmental or severely comminuted fractures in high-energy trauma. Because of the problems and the classification of type 3 injuries, this group was subsequently divided in three subgroups. Type 3A injury has a large soft tissue laceration or flaps but allows for adequate soft tissue coverage of bone. Also included in type 3A are fractures with severe comminution or segmental fractures, regardless of the extent of the soft tissue damage. Type 3B fractures (Fig. 17-3) are more severe, and they have extensive periosteal stripping and soft tissue loss with significant bone exposure and massive contamination. Type 3C fractures have an arterial injury requiring repair. A more comprehensive soft tissue scale, albeit more difficult to use, is the AO soft tissue scoring system.94 It incorporates five grades of severity and three categories of tissue. The AO classification grades the skin, muscle and tendon, and neurovascular structures. Closed fractures involving only skin are graded in four subgroups. For open fractures, four grades are given. A new feature of this classification is the evaluation for muscle and tendon injuries. Because of the prognostic value, knowledge of the extent of muscle damage and tendon involvement is essential. A common approach in all classification schemes is a determination of the length of laceration of the skin. As treatment methods have become more comprehensive and more systemic factors are taken into account when treating open fractures, the presence or absence of muscle injury, nerve injury, and vascular injury has become more important prognostically. Acute systemic factors, such as shock, associated injuries, or older age, have been recognized as important prognostic indicators also. They influence the acute treatment of fractures and the treatment of complications. For example, in osteomyelitis, debilitating factors, such as smoking and malnutrition, affect the feasibility of reconstruction.12 Ruedi et al108 have developed a classification system that characterizes soft tissue injury by addressing several layers of the soft tissue envelope. This classification system determines whether the integument is open or closed. Injuries to muscle, tendon, nerve, and vessels are graded in order of severity. Although this may be more complex than the Gustilo and Anderson43 classification of open fractures, it is an attempt similar to Tscherne’s116 classification to define in more depth the deficiency and defects of the soft tissues. Factors such as contusion or ecchymosis to skin and muscle must be identified to prevent further damage to these tissues in surgical dissection. Such damage of muscle or fascia territories can make these sites unreliable as replacement tissue. The abilities of the reconstructive surgeon, particularly the ability to transplant autogenous tissue such as muscle or skin flaps, have changed the concept of debridement.39 Surgeons treating combined injuries must accept the premise that irreversibly damaged or nonviable tissues require replacement, and the zone of injury requires expeditious reconstruction. Marginally viable tissue left behind can subsequently desiccate, infarct, and become infected, adding further delay in healing. This results in progressive dysfunction related to inflammation, fibrosis, and pain, which can be avoided if aggressive debridement is undertaken primarily. Critical vascular structures, nerves, and tendons can be cleaned, and prompt coverage can preserve their viability. Debridement may take place in the acute trauma setting or in a chronic wound that has evolved from improper handling of soft tissues. Other devices, such as the Versajet Hydrosurgery System (Smith & Nephew, Hull, England), enables the surgeon to cut and remove damaged tissue and contaminants while simultaneously irrigating the wound. Surgical debridement is accomplished in a single step. The Versajet (Fig. 17-4) uses a high-velocity stream of sterile saline that jets across the hand piece into a vacuation collector. The Versajet requires less irrigant than traditional techniques, and it confines the irrigant to the wound area. In the acute trauma situation, this obviates the need to change large saline irrigant bags and reservoir waste canisters. This system has several power settings that can be used depending on the degree of debridement needed. One advantage of the hydrosurgery system is that there is less aerosolization of bacteria, which decreases risk to the operating room staff and surgeon based on a single wound site. Compared with conventional pulsate irrigation, the Versajet leaves significantly less bacteria in the wound. Swiontkowski114 has popularized the use of the laser Doppler to determine bone blood flow in planning for debridement of infected bone. All areas of the bone not covered with periosteum are removed, and those that are exposed are burred with an iced saline bur. If punctate bleeding is encountered from the cortical bone, the bone is left behind. If not, the bone is removed until the paprika sign is identified. That is a sign of bleeding bone and live bone. This is punctate bleeding from the haversian canals that indicates bone viability. If a sequestrum is in the medullary canal, the anterior part of the bone cortex (such as the metatarsal) should be removed to provide a window for access to the medullary canal for placement of muscle flaps, which eliminates dead space and helps control infection. 1. The extremity is prescrubbed to remove grime and surface dirt, and then it is prepared and draped. Nails should be cut and nail plates cleaned. 2. The leg is elevated for 5 minutes to exsanguinate it (rather than wrapping the extremity with an Esmarch bandage), and the tourniquet is inflated. 3. The wound is superficially washed with an antibiotic-saline solution to remove blood clots and superficial debris. It is advisable to use loupe magnification when debriding. The more complex the wound, the longer the debridement takes. If the patient with an open wound has been waiting for quite some time and blood is organized in muscle tissues or around fracture ends. Half-strength peroxide can be used as a first rinse solution to lyse the clot and gain access to the true depths of the wound. Half-strength peroxide has a tendency to bubble and should be washed away with normal saline solution. 4. A No. 10 or 15 scalpel blade or very sharp scissors is used to excise the skin and dermis, particularly around the edge of the wound, back to normal tissue. 5. The subcutaneous layer is inspected and debrided sharply with a No. 15 scalpel blade to the level of fascia. All fascia that is stripped, avulsed, or contaminated should be removed. 6. The next layer encountered is muscle. Muscle should be resected down to healthy tissue, regardless of the amount of muscle removed. Leaving unhealthy necrotic muscle is one of the surest ways to initiate an infection. 7. Periosteum that is elevated from bone should be excised to the level from which it is elevated. Small bone fragments devoid of periosteum or free-floating large segments, even structural ones, should be removed for fear of colonization, contamination, and infection. 8. At the conclusion of debridement, the wound is again irrigated. The tourniquet is released and all tissue planes, particularly the muscle, are observed for bleeding as the arterial pressure increases within the limb. 9. Areas that remain nonviable, particularly the dermis, skin, and muscle, are reexcised. Excision then can be done sequentially, watching for punctate bleeding from the dermis or the muscle. When large flaps have been avulsed, excision is carried out through the skin to the level of bright red blood coming from dermis on incision. 10. No attempt should be made to close the wound defect under any tension, for fear of further ischemic damage to already compromised tissue. The optimal time for soft tissue reconstruction in severe open fractures remains controversial. The argument favoring a staged method is the need for a second-look debridement. If there is uncertainty about traumatized and devascularized tissue, a second look is performed. The main argument for early reconstruction is to reduce the nosocomial contamination and secondary necrosis of exposed tissues. Late soft tissue reconstruction is associated with a significantly higher infection rate and flap complication rate when compared with early (within 72 hours) soft tissue coverage.20,36 Godina40 and other pioneers64 changed the concept of primary repair and reconstruction of damaged tissue by advancing the phase of reconstruction from a delayed elective procedure to the day of injury. Assuming that an adequate primary debridement is feasible, the outcome should be improved by immediate soft tissue closure to avoid bone infection. Immediate reconstruction improves the time to definitive fracture union, decreases the number of operations that are performed, and reduces infection rates.56 Patients with IIIB and IIIC open fractures of the foot and ankle whose general condition was suitable for debridement, followed by stable internal fixation and immediate soft tissue reconstruction, demonstrated a better outcome and a shorter period of convalescence.56 The higher incidence of infection in the delayed group may well be due to the lengthy exposure of the fracture to nosocomial contamination, the secondary damage of exposed tissue, or the necessarily incomplete nature of second-look debridement, particularly in and around a reduced fracture. There are four general types of newer wound dressings: semipermeable films (e.g., OpSite, Tegaderm [3M, St. Paul, Minn.], and bio-occlusive), hydrogels (e.g., Vigilon), occlusive hydrocolloids (e.g., Duoderm, Synthes, West Chester, Pa.), and synthetic skin substitutes (e.g., Epigard [Synthes, West Chester, Pa.] or Integra [Integra LifeSciences Corp., Plainsboro, N.J.]).34 These newer dressings are best applied when the wound site is surrounded by a border of healthy tissue. Newer dressings are not without drawbacks, however, particularly the accumulation of exudate, hematoma, and seroma beneath them.34 In addition to these wound dressings, there is an isolated report on the efficacy of honey as a broad-spectrum antimicrobial found to be effective in controlling Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Klebsiella pneumoniae. Although this finding needs further clinical verification, honey shows promise for use as a first-line wound dressing agent.52 Antibiotic beads have been used effectively to prevent or to control infection and can be used after the first debridement until the patient returns to the operating room for a second-look debridement (Fig. 17-5). Advantages of antibiotic beads are that they can deliver antibiotics at high concentration to a compromised wound without systemic effects. The bead pouch, popularized by Henry et al,55 seals the wound so that transudate from the wound surface is captured, bathing exposed tissues in physiologic fluids that also contain bactericidal levels of antibiotics. Wounds do not desiccate, cell death is avoided, and infection risk is reduced. The technique of local antibiotic therapy achieved via antibiotic-impregnated polymethyl methacrylate (PMMA)54 originated with Büchholz in Germany. It was an application that used antibiotic-impregnated bone cement to treat infected arthroplasties.53 Antibiotic-impregnated PMMA beads are strung on steel surgical wire. A chain of medium-sized 6.3-mm PMMA beads is composed of 21 beads, each bead weighing 70 mg and containing a 5.7-mg tobramycin base. A chain of smaller beads has 20 beads that are 2 mm in diameter, each weighing 14.5 mg with a 2.2-mg tobramycin base. The surgical wire consists of three strands of size 2-0 surgical wire for the 6.3-mm beads. With the smaller size, four strands of 4-0 steel sutures are used.123 The custom-made versus commercial fabrication processes differ in that the custom-made beads are polymerized in a mold, whereas the commercial variety is formed in a press with a corresponding increase in temperature and pressure. The bead pouch technique is most effective for grade III open fractures. It is indicated for use in grade I fractures only if primary closure was prevented because of compartment syndrome, marked swelling, or wound edema.54 The bead pouch technique is performed in the operating room under sterile conditions. 1. All necrotic, avascular, and contaminated tissue is removed from the fracture site during the initial irrigation and debridement. Wound margins are extended to appropriate widths. 2. A thorough lavage consisting of bacitracin and normal saline is performed.117 3. Depending on the severity of the fracture, reduction is accomplished with either external or internal fixation. 4. One or more chains of antibiotic beads are inserted into the wound surrounding the fracture site. Placement should be such as to fill the soft tissue cavity but leave adequate room for closure. 5. A suction drain (0.32-cm diameter) is placed in the wound. The drain should be positioned so that it exits the hematoma site through normal tissue.117 6. If possible, the wound should be closed with interrupted sutures. (In wounds with extensive soft tissue damage, closure may not be possible at the time of initial debridement.) 7. Wound coverage is achieved with an adhesive polyethylene wound film, such as OpSite. The semipermeable wound dressing should be stapled to the skin edges and a second layer wrapped around the entire wound area to prevent leakage of wound secretions. 8. The drain should remain in place for 48 hours. Suction is avoided because it would negate the high bactericidal dosages released by the beads into the wound hematoma. 9. The bead pouch is replaced every 48 to 72 hours in the operating room under sterile conditions. This is done to ensure adequate antibiotic concentrations in the wound environment. Aerobic and anaerobic cultures are taken at each bead change. Final wound closure is achieved through either primary suture closure or, in cases with more extensive soft tissue defects, split-thickness skin grafts or flap coverage. In more severe fractures, the bead pouch provides a solution to much of the debate surrounding delayed or acute soft tissue transfer. Because the bead pouch delivers high levels of antibiotics locally, the surgeon can delay definitive coverage until thorough and usually multiple debridements have been performed, a clean wound is achieved, and operative repairs of neurovascular, tendon, and ligamentous structures are made.54 If an acute flap is indicated, the presence of the bead pouch beneath the flap serves to assuage the fears the surgeon might have that infection could occur beneath the flap. Hyperbaric oxygen (HBO) can be used to promote formation of granulation tissue and stimulate angiogenesis in wounds that are compromised, usually by impaired arterial inflow or venous outflow.66 In addition to exposure to hyperbaric oxygen, wound dressings may be changed under sterile conditions by chamber personnel under sedation, avoiding discomfort to the patient. Patients who have gas gangrene associated with fractures require emergent debridement, hyperbaric oxygen, antibiotics, and ultimately fracture and soft tissue management.91 Normal tissue oxygen levels are approximately 40 mm Hg. When tissue levels fall below 30 mm Hg, normal metabolic activity is significantly impaired.42 In infected wounds and traumatized tissue, oxygen levels often fall to less than 30 mm Hg. HBO enhances oxygen delivery to ischemic and hypoxic wounds, and even when it causes local vasoconstriction, the overall increase in blood oxygen content results in a net gain so that the net oxygen concentration at the wound increases. HBO improves neutrophil function, facilitates fibroblast cell division, increases collagen formation, and encourages new capillary budding. The promotion of angiogenesis by HBO is thought to be one of the major factors in promoting the healing of chronic hypoperfused wounds.59 Negative pressure therapy (NPT) exposes a wound to subatmospheric pressure. It has proved to be extremely effective in treating a wide spectrum of wounds, including traumatic wounds as well as dehisced incisions with or without exposed hardware.6 The wound cavity is dressed with a cell foam dressing that is connected to an adjustable vacuum source with a negative pressure of up to 125 mm Hg. The foam dressing and wound site are sealed with a thin adhesive film, converting the open wound to a controlled closed wound. Pressure is applied continuously or cyclically to the wound (Fig. 17-6). The removal of excess interstitial fluid from the wound periphery results in a decrease in the local interstitial pressure, thus restoring blood flow to compressed or collapsed vessels. Along with removal of the chronic fluid, factors that inhibit healing are also removed. An additional mechanism of action of NPT is the mechanical stimulation of cells by tensile forces placed on the tissue because of the collapse of the foam dressing by the negative pressure. In complex extremity injuries, the treating physician must first determine whether limb salvage is feasible. Before complex and prolonged reconstructions are started on a limb that will ultimately function poorly or not at all, a well-fitted prosthesis should be seen as an excellent therapeutic option, and early amputation should be considered. Lange73 and Hansen47 have delineated a sound algorithm for these difficult cases. Although the evolution of sophisticated microsurgical reconstructive techniques has created the possibility of successful limb salvage in even the most extreme cases, such technical possibilities are double-edged swords. Hansen,47 in analyzing his vast personal experience with managing open fractures, noted that protracted limb salvage attempts might destroy a patient physically, psychologically, socially, and financially, with adverse consequences for the patient’s family as well. In spite of the best attempts, the functional results of limb salvage are often worse than those of an amputation. Thus enthusiasm for limb-salvage techniques, especially of the traumatized foot and ankle, must be tempered by a realistic assessment of the results, not just for the injured part but for the patient as a whole.73 A salvaged limb must function as well as, if not better than, a prosthesis, or heroic attempts at reconstruction are not indicated. Donor-site morbidity should also be considered with free tissue transfer when considering limb reconstruction. Similarly, a given tibial injury might need to be treated differently if there is unreconstructible ipsilateral foot trauma that precludes reasonable limb function even if the leg is salvageable. According to several authors, complete disruption of the posterior tibial nerve (in association with type IIIC tibial fracture) is also a functional liability significant enough to warrant amputation.21,58,74 Decreased sensation on presentation is not an indication for amputation. Expedient amputation of a massively traumatized limb, even if it appears salvageable, may also be necessary in the multiply injured patient who cannot tolerate the reconstructive time or metabolic demands of the reconstruction. This is an extremely difficult judgment that is highly individualized and impossible to quantitate.77 The skin is composed of dermis and epidermis. The dermis contains sebaceous glands, most of which are appendages of hair follicles.107 Sweat glands and hair follicles are mostly located in the deep dermis. These skin appendages are lined with epithelium, thus allowing for re-epithelialization after removal of epidermis and partial removal of dermis, as in superficial burns or harvesting of split-thickness skin. 1. The donor site is prepared by first removing any povidone-iodine and other foreign materials from the skin. Mineral oil is applied liberally to both skin and dermatome to aid in gliding. 2. The dermatome is turned on, placed at a 45-degree angle with the skin, and advanced firmly to prevent slippage. As the graft is being harvested, punctate bleeding from a white dermal bed should be seen. If subcutaneous fat is noted, harvesting should be stopped immediately, the graft replaced, a new donor site chosen, and the thickness setting on the dermatome readjusted. 3. After an adequate amount of skin has been harvested, the dermatome is lifted from the donor area. 4. The donor area is temporarily covered with a hemostatic agent (local anesthesia with epinephrine or topical thrombin is a popular choice). (Donor-site dressings are reviewed later in this discussion.) 5. The skin graft is then meshed. Meshing is necessary for two reasons. First, STSG failure is usually secondary to hematoma formation beneath it, and meshing of the skin allows drainage postoperatively. Second, when a large amount of graft is needed (e.g., for a large burn), meshing also allows the graft to expand so that a relatively small piece of skin can cover a larger area. Most popular meshers require placement of the skin on a “carrier” chosen for the ratio of mesh. Unless a paucity of STSG donor sites requires a larger ratio, most grafts are meshed at 1 to 1.5. Disadvantages of meshing mostly relate to the aesthetics of the healed meshed skin, which retains its “meshed” appearance in the long term (Fig. 17-7).
Soft Tissue Reconstruction for the Foot and Ankle
History
Complex Musculoskeletal Injuries
Open Fractures
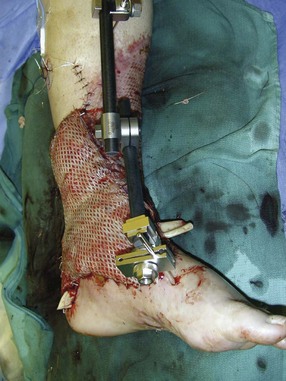
The Reconstructive Ladder
Classification of Soft Tissue Injury
Debridement
Chronic Wounds
Debridement Technique
Surgical Technique
Timing of Reconstruction
Preparation of the Wound before Reconstruction
Wound Dressing
Antibiotic Beads
Antibiotic Bead Pouch
Surgical Technique
Discussion
Hyperbaric Oxygen
Negative Pressure Therapy
Amputation versus Salvage
Wound Closure
Skin Grafting
Split-Thickness Skin Grafting
Surgical Technique
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree