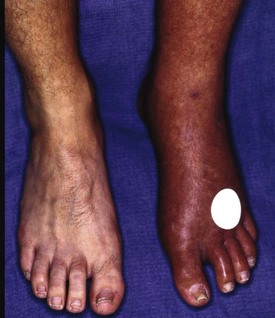
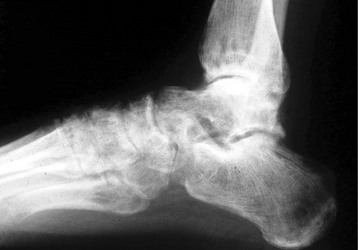
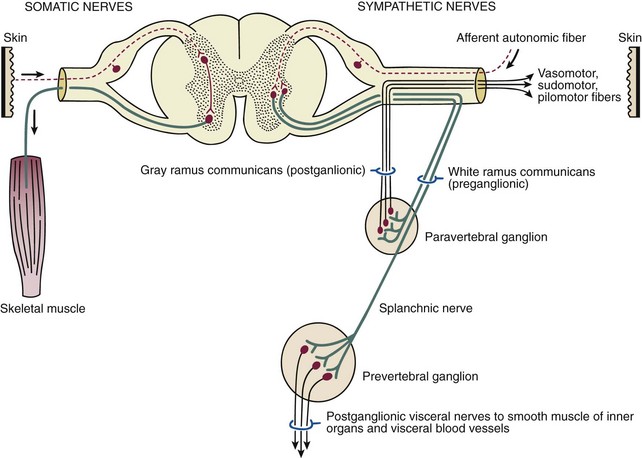
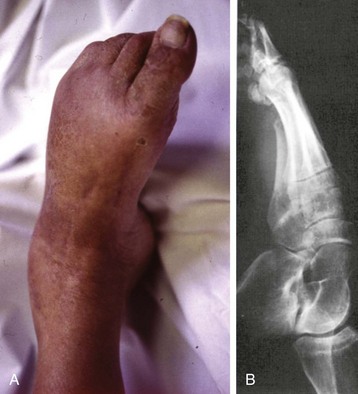
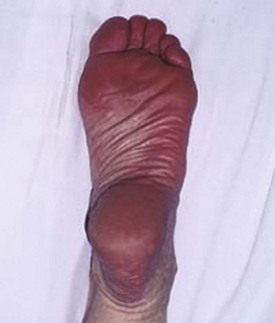
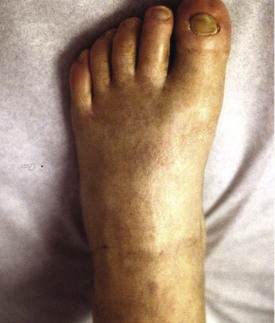
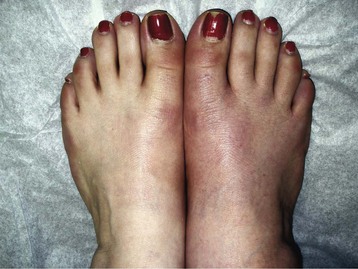
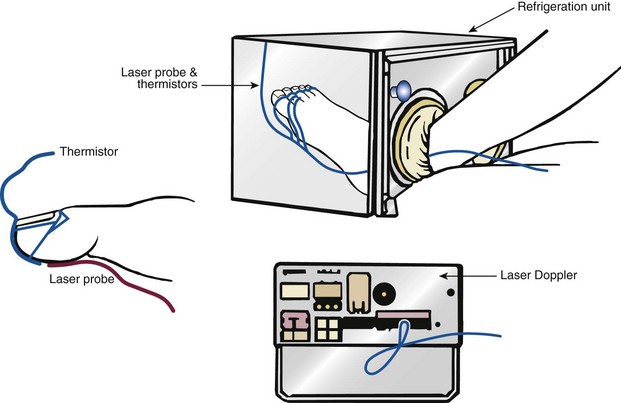
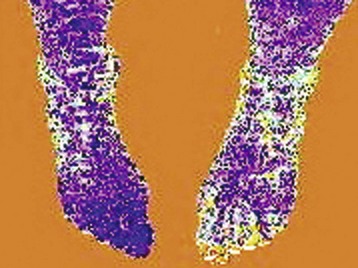
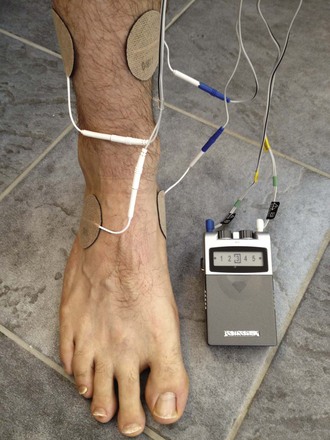
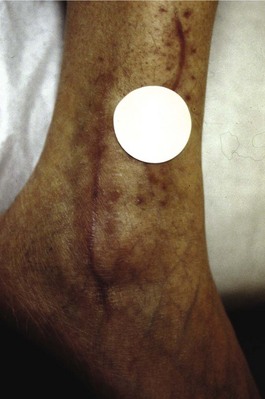
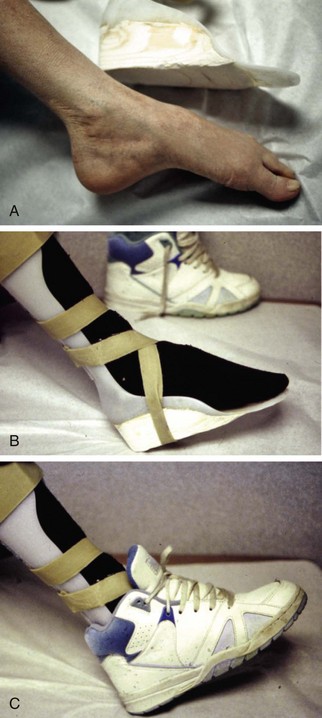
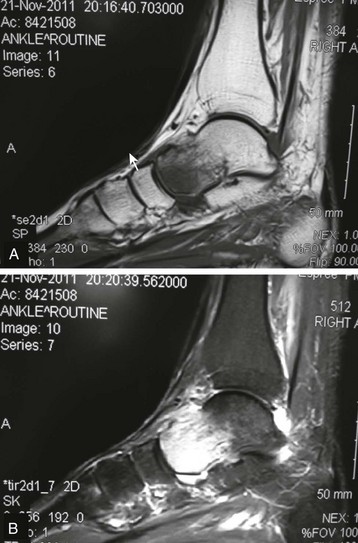
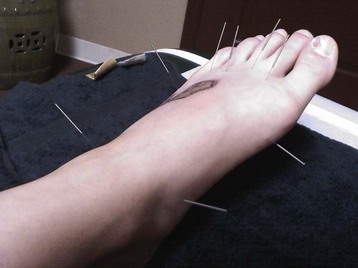
Chapter 14 Complex regional pain syndrome (CRPS) has historically gone by many names, including sympathetic trophoneurosis, minor causalgia, Sudeck atrophy, algoneurodystrophy, acute bone atrophy, peripheral trophoneurosis, painful osteoporosis, sympathalgia, and its most well-known former name—reflex sympathetic dystrophy (RSD). The term reflex sympathetic dystrophy (RSD), coined by Evans in 1946, was initially the popular descriptive term and remains part of the vocabulary of many treating physicians. Reflex sympathetic dystrophy describes a symptom however, and not a disease state. Complex regional pain syndrome, put forth by the International Association for the Study of Pain (IASP) in 1993, is now the accepted term.9 Historically, in the seventeenth century, the French battlefield surgeon Ambrose Paré first described this syndrome of burning pain after peripheral nerve injury. King Charles IX was treated for smallpox by Paré, who lanced his arm. After treatment, the king suffered from persistent pain, muscle contracture, and inability to flex his arm. In 1864, Mitchell, an American Civil War physician, coined the term causalgia for the burning pain that follows gunshot injuries to the peripheral nerves.56 In 1900, Sudek observed spotty radiographic osteopenia in affected extremities. A little later in 1916, Leriche implicated the sympathetic nervous system, and subsequently, Livingston (1943) described the vicious circle of pain. This theory described abnormally firing self-sustaining loops in the dorsal horn. These were thought of as small irritation foci of nerve endings of major trunk nerves, which activated central projecting fibers, giving rise to pain. There is controversy about the value of the consensus process in this setting. There has been an almost complete absence of evidence-based information about this condition since it was newly defined. According to the IASP, complex regional pain syndrome exists in two forms. CRPS I is an inappropriate nonspecific pain syndrome occurring after trauma. CRPS II develops after an injury to a specific nerve. CRPS type I (formerly RSD) develops after an initiating noxious event. It features a triad of sensory, autonomic, and motor symptoms that occur distally in the affected extremity in a generalized distribution. The symptoms are independent of the type and location of the preceding trauma. The symptoms are not limited to the distribution of a single peripheral nerve and are routinely disproportionate to the inciting event. To understand this pathologic entity thoroughly, one must become familiar with a new vocabulary of terms used to describe altered sensations (Box 14-1). Three prerequisites are needed to define CRPS: a painful lesion, an abnormal autonomic reflex, and an inherent tendency or predisposition that renders the patient susceptible to developing the syndrome (i.e., after trauma, burn, surgery, etc.) (Table 14-1). Onset may occur immediately (in the recovery room) or days to weeks after the initial trauma but typically is present within 1 month of the noxious event. A diagnostic delay of 6 to 12 months is not unusual in the lower limb. Classic stages of RSD are no longer used to classify the progressive development of the disease.6,9 Table 14-1 IASP Diagnostic Criteria for Complex Regional Pain Syndrome (CRPS)* 1. The presence of an initiating noxious event or a cause of immobilization.† 2. Continuing pain, allodynia, or hyperalgesia in which the pain is disproportionate to any known inciting event. 3. Evidence at some time of edema, changes in skin blood flow, or abnormal sudomotor activity in the region of pain (can be sign or symptom). 4. This diagnosis is excluded by the existence of other conditions that would otherwise account for the degree of pain and dysfunction. IASP, International Association for the Study of Pain. *If seen without “major nerve damage,” diagnose CRPS I; if seen in the presence of “major nerve damage,” diagnose CRPS II. †Not required for diagnosis; 5%-10% of patients will not have this. Modified from Besse JL, Gadeyne S, Galand-Desme S, et al: Effect of vitamin C on prevention of complex regional pain syndrome type I in foot and ankle surgery, Foot Ankle Surg 15:179–182, 2009. CRPS almost always includes inappropriate pain.59,83 The pain is deep, spontaneous, burning, and diffuse. Pain can be triggered by a breeze, the lightest touch of a physician’s hand, or even certain emotionally laden words. Patients often describe some combination of the skin feeling raw, an electric-like shooting pain, or a deep, dull bone ache. Cutaneous and deep hyperpathia are typically found. Allodynia is present, depending on the time interval from the initiating event. The pain may be sympathetically maintained, sympathetically independent, or a combination of the two. After exercise, the discomfort usually worsens. Autonomic changes consist of swelling (edema), abnormal vasodilation, skin warming, and changes in sweating pattern (e.g., hyperhidrosis or hypohidrosis) (Fig. 14-1). Vasoconstriction with associated trophic changes come later in the disease. Motor changes demonstrate decreased active range of motion, decreased strength, and increased physiologic tremor (dystonia) of the distal extremity. There may be difficulty in initiating motion. Sensory changes include hypoesthesia, hyperesthesia, allodynia, and anesthesia dolorosa. Trophic changes (e.g., shiny skin, nail changes, lack of hair growth, contractures, muscle atrophy, osteoporosis, and cool, pale skin) are late findings (Fig. 14-2). A review of current literature suggests that CRPS I has a very complicated pathophysiology at several integrated levels of the entire neurologic pathway. Changes can involve the somatosensory, sympathetic, and sudomotor systems, which result in the imbalance between inhibitory and excitatory nerve function (Fig. 14-3).70 In the neuropathic state, peripheral areas of the nervous system not normally responsible for pain transmission are altered and now conduct pain. A-β fibers, normally pathways for touch, start to sprout and grow into the C-fiber terminals, becoming fast transmission conduits for pain.3 Nerves outside the main area of injury may be recruited, thus explaining the late development of pain in peripheral, noninjured anatomy. With chronic central sensitization, afferent sensory information is relayed to the cerebral cortex via the thalamus. The chemicals released from the damaged tissue (histamine, substance P, bradykinins, prostaglandins, serotonin, acetylcholine) centrally and peripherally remain elevated, and neuronal plasticity develops. At this point, the structure, function, and biochemical profile of nerves actually modify.19 Subsequent to these biomechanical abberations, abnormal feedback mechanisms develop. Normally dormant genes that encode ion channels, receptors, and neurotransmitters are activated,23 causing sympathetic tone to persist inappropriately.36,43 With chronic pain, cortical reorganization of the representation of the extremity in the central nervous system (CNS) can also take place.49 The autonomic nervous system controls thermoregulatory and nutritional blood flow. Inappropriate arteriovenous shunting and abnormal receptor input can cause vasomotor, temperature, and trophic changes. A hot, swollen foot and a cold, ischemic, stiff foot can both be explained by this mechanism (Fig. 14-4).42,43 Skin blood flow and sweating abnormalities are also usually restricted to the nerve territory. Motor function deficits are explained by injury to motor axons. Swelling and trophic changes are discrete. Peripheral nerve changes are responsible for the CRPS II pathophysiologic phenomena.2 Sympathetically maintained pain (SMP) or sympathetically independent pain (SIP) may be a component of either syndrome, and symptoms of CRPS I and CRPS II may be present in the same patient. Animal research using sympathetic blocks, α-adrenergic receptor agonists, and electrical stimulation suggests that sympathetic nervous activity can be altered to produce pain. In the diabetic patient, the neuropathic painful foot has selective sympathetic denervation, but the neuropathic painless foot does not. The degree of pain is altered by the intensity and ongoing nature of the stimulus, afferent input, efferent modulation, CNS interpretation, and physiologic adaptation.31 Good evidence indicates that true CRPS I or II is not psychogenic. No personality disorder is specific to CRPS. Severe pain of the disease complex is the direct cause of the psychologic suffering, not the converse.9,17 One can often see anxiety, hopelessness, and depression in the patient. However, there is no question that other factors, such as fatigue, emotional state, stress, gender, and culture, have been shown to affect pain transmission. Genetics also might play a role, with the possibility that CRPS is a neuroimmune disorder linked to multiple sclerosis and narcolepsy.25,51 Prompt recognition can prevent unnecessary investigations and lead to treatment that will shorten the course of the disease. The differential diagnosis includes: Erythromelalgia is a poorly understood clinical syndrome manifesting as hot, red, painful extremities, usually affecting the lower limbs. It is classified as primary (idiopathic) or secondary, following myeloproliferative syndrome–related thrombocytopenia. Cold, elevation, and a daily dose of aspirin usually provide relief (Fig. 14-5). Sensory examination is important to assess the location and distribution of the neuropathic pain and to determine if it involves a single or multiple dermatomes. One must initially establish if there allodynia (mechanical and thermal), hyperalgesia (mechanical and thermal), paresthesias, or any other pathologic neurologic signs (Fig. 14-6). Motor evaluation must assess atrophy, muscle strength, joint motion and stability, gait, and coordination. Some patients have edema with decreased range of motion, and the involved extremity may be warmer or colder, with or without hyperhidrosis. Changes in nail and hair growth patterns are commonly observed and should be looked for. In a study by Veldman78 of patients with CRPS, discoloration of the skin was reported in 91%, altered skin temperature in 92%, edema in 69%, and decreased range of motion in 88% of patients (see Fig. 14-6). The area of the body that has been exposed to chronic pain will gradually decondition. Normal symmetric coordinated motion ceases. Immobility leads to obesity, which further overloads the painful site. To compensate, the patient overuses adjacent normal areas. Further arthropathies and myopathies develop. A progression from cane to walker to wheelchair is not uncommon (Fig. 14-7).23 Although reliance on a single diagnostic test for CRPS can be misleading, and clinical evaluation is the mainstay of diagnosis, a three-phase technetium bone scan is the prime imaging technique for the disease. Diffuse increased tracer uptake in the delayed phase (phase 3) is said to demonstrate increased bone metabolism. Phases 1 and 2 are not specific for CRPS. Bone scan is reported to be 96% sensitive and 98% specific; other studies have shown it to be only 44% sensitive. Localized uptake might give clues to an underlying precipitating injury (Fig. 14-8). For CRPS type I, the test seems to be more reliable when done within 6 months of the onset of symptoms and in patients older than 50 years.7,20,28 A negative bone scan in the early phases of the disease is quite common. Despite intensive study, however, the physiologic significance of bone scanning is still unclear. Heat, cold, and touch sensitivity can be assessed with a quantitative thermal stimulator, von Frey hairs, or electrical stimulation (Table 14-2). Table 14-2 Simple Bedside Sensory Testing for Complex Regional Pain Syndrome Types I and II* *Compare the opposite side for each test. From Gracely RH, Price DD, Roberts WJ, et al: In Jánig W, Stanton-Hicks M, editors: Reflex sympathetic dystrophy: a reappraisal. Progress in pain research and management, vol 6, Seattle, 1996, International Association for the Study of Pain, pp 151. Plain radiographs and computed tomography (CT) are not specific. Plain radiographs can show patchy demineralization at 3 weeks, usually more than would be expected from disuse alone. Magnetic resonance imaging (MRI) can show bone marrow stress reaction earlier than the bone scan (Fig. 14-9). Figure 14-9 A and B, Underlying talar contusion on magnetic resonance image rules out complex regional pain syndrome. Attempts to block α-adrenergic receptor activity are not entirely reliable or reproducible and may be fraught with the problems of placebo effect and false-positive results. Despite these limitations, response to the block indicates that sympathetically maintained pain is involved. Use of blocks is therefore important for establishing the diagnosis of CRPS and planning treatment (Table 14-3). The IASP and others no longer regard differential spinal blockade to be reliable. At best, it can be considered a screening test of sympathetic function.71 Nerve blocks must be evaluated in terms of the entire clinical picture (e.g., decreased pain, temperature increase of 3° F or more, venous engorgement, change in skin color). Therefore sympatholysis, whether pharmacologic or by nerve conduction block, is critical in disease determination. Pain conditions without any response to sympathetic block are defined, by contrast, as sympathetically independent pain states. Isolated cold stress testing (cold stressor test), combined with thermographic imaging, can measure total digital (great toe) blood flow and microvascular perfusion (Fig. 14-10). The procedure combines a stressor (refrigerated environment) with a measurement technique and can provide accurate assessment of autonomic and vasomotor stability and the effectiveness of sympathetic blocks.28,44 Limb surface temperature differential of 1° F or more is thought to be significant (Fig. 14-11).4,25,51,70 Electromyographic (EMG) and nerve conduction velocity (NCV) testing should be done together if an underlying discrete nerve lesion is suspected. This is critical to the diagnosis of CRPS II. Unfortunately, insurers might rule out this diagnosis because results of these studies are negative. These tests measure large-fiber disease. The critical distinction to make is that much of neuropathic pain is small-fiber (C-fiber)–mediated pain and that small-fiber abnormalities are not well tested with EMG. Therefore EMG/NCV testing has some internal inconsistencies (Fig. 14-12). When using physiotherapeutic maneuvers, it is best to minimize joint movement in the affected region to reduce the mechanoreceptor barrage and its increase in perceived pain.70 One should begin with gentle gliding exercises. One should emphasize to the therapists, even by including it in the prescription, that forcing patients beyond their tolerance can worsen the syndrome. A transcutaneous nerve stimulator (TNS) can help reduce pain and allow the patient to progress, especially in patients in whom the pain is limited to one dermatome. This has been shown to release endorphins (naturally occurring morphine-like substances that relieve pain) and can block nerves with benign impulses, preventing their use for pain transfer (Fig. 14-13). Alternative medicine in the form of acupuncture is receiving new interest. It has been part of Chinese medicine for 2500 years and is based on the general theory that patterns of energy (Qi, pronounced “chee”) flow through the body and are essential for health. All body parts are divided into yin and yang. In the human body, tendons and bones are yin and the skin is yang. Harmony and good health occur when the yin and yang are in perfect balance. Disruptions of this balance cause disease by changing the flow of Qi, blood, and body fluids.47 Acupuncture points are located where the Qi moves to the surface of the body (361 points). Insertion of the needles at the appropriate points causes a stimulus to travel to the spinal cord, releasing enkephalin and dynorphin (central neuropeptide endorphins) that attenuate pain transmission (Fig. 14-14). According to the gate control theory of pain, the stimulation of large-fiber transmission during treatment “closes the gate” to pain by overwhelming the nerve’s transmission capability. Stimulation is controlled by the length and depth of insertion, the shape of the needle, and needle stimulation (heat, electricity, vibration, and rotation). Figure 14-14 Acupuncture on the right foot to help treat allodynia resulting from tatoo removal treatments. Electroacupuncture has provided relief of pain, vasodilation, and erythema, even in some refractory cases. Treatments are usually done at low frequencies (less than 10 Hz) for 20 minutes. Unfortunately, the relief from acupuncture is usually transient, and reimbursement for the procedure limits access to it.53 In adults with suspected CRPS, nerve blocks should be given early. These syndromes can partially respond to sympathetic nerve blockade. Increased limb temperature and at least 50% relief in pain after the block are good signs (Fig. 14-15). Neural blockade can provide sympathetic pain relief much longer than pharmacologic agents can. If associated motion loss and trophic changes are present, physical therapy, especially during the pain-free period provided during the block, is essential. Blocks may be used as often as every other day for 2 weeks to achieve long-lasting effect.7,46 The use of opioids (compounds with morphine-like characteristics) for the treatment of neuropathic pain is no longer controversial and should be part of your armamentarium. Neuropathic pain is not opiate insensitive, and it was suggested at the Fourth World Congress of The World Institute of Pain, Budapest, 2007 that these drugs be titrated for therapeutic efficacy versus side effects. Opiates have the side effects of nausea, constipation, respiratory depression, urinary retention, orthostatic hypotension, confusion, and sedation, but these can be managed clinically. Opioids also inhibit rapid eye movement sleep, which can disturb sleep patterns.18 Lowering the dose of opioids whenever possible is advisable. However, modern pain management recognizes that there is no ceiling to the therapeutic dose of opioids. As stated by Tennant,72 “There is no upper limit on the amount or number of different opioids prescribed for pain relief as long as the administration of the medicine does not produce physiologic abnormalities as diagnosed by face-to-face, hands on physical examination.” Current clinical practice encourages the use of opiates to cover all of the painful time as opposed to on an as-needed basis. There should be fixed doses of long-acting medication (e.g., transdermal fentanyl patch, MS Contin, OxyContin), with the ability to supplement with additional short-acting medicine for breakthrough pain (oral transmucosal fentanyl, oxycodone, hydromorphone, or hydrocodone).64 In the elderly, long-acting opiates may be more efficacious than tricyclics. One placebo-controlled randomized clinical trial (n = 43) was found that investigated the effects of sustained-release oral morphine on patients who had previously been treated with epidural spinal cord electrical stimulation (ESES).34 No significant differences were found between the extent of pain reduction and the average time for ESES to become effective. On average, the morphine group reported 20 side effects per day, compared to 2 per day in the placebo group. An uncontrolled study with nine CRPS I and II patients evaluated the effects of continuous infusion of morphine in the axillary plexus after stellate blockade.5 Significant pain reduction at rest and at movement and increased grip strength were found. However, the steady-state morphine concentrations were lower than the minimally effective analgesic concentration. There is little information on weak- and strong-acting opioids in patients with CRPS I. Systematic reviews on their use for neuropathic pain have found tramadol to be effective.22 Positive short-term effects have also been reported for strong-acting opioids administered for neuropathic and musculoskeletal pain.35 Atypical opioids such as tramadol, an opioid receptor agonist, are clearly different from other opioids and are only minimally inhibited by naloxone. Tramadol inhibits the uptake of serotonin and norepinephrine and has been shown to be effective in some patients with neuropathic pain. It has a low potential for abuse, but it should be avoided in patients with a tendency to abuse drugs.7 The use of paracetamol is described in the context of an adjuvant pain protocol in a study about the efficacy of free radical scavengers in treating CRPS I (n = 146).63 Sixty-one CRPS I patients were retrospectively evaluated with respect to the effects of 60 mg of ketorolac administered by means of a regional intravenous blockade.14 Twenty-six percent of patients had a complete response, 42% had a partial response, and 31% had no response. Patients with allodynia had significantly less response to the treatment. Conflicting data have been published with regard to the use of NSAIDs in patients with neuropathic pain.58 In considering the use of antidepressants, four major issues must be considered: 1. Major depression and chronic pain are common conditions, and they frequently overlap. 2. Antidepressants can improve symptoms of major depression, regardless of the presence or absence of comorbid pain (although pain can reduce the chances of optimal recovery). 3. Antidepressants improve pain symptoms, regardless of the presence or absence of comorbid major depression. 4. Chronic pain and major depression have a shared neurobiology and appear to have a shared neuroanatomy (in the brain and spinal column) and neurochemistry (norepinephrine and serotonin), with similar hypothalamic-pituitary-adrenal (HPA) axis, autonomic nervous system (ANS), and inflammatory cytokine disturbances. Tricyclic antidepressants (TCAs) and selective serotonin reuptake inhibitors (SSRIs) have more benign side-effect profiles than classic analgesics and offer the added benefit of often restoring sleep.25,40,55 TCAs, especially amitriptyline (Elavil), work by blocking norepinephrine and serotonin reuptake, thus making more neurotransmitter available. They might also block the NMDA receptor. Amitriptyline also is a sodium channel blocker and imparts a local anesthetic-like quality. Monoamine oxidase inhibitor (MAOI) use should be reserved for experienced clinicians in special situations, because their complications are potentially fatal (Box 14-2). Oral or intravenous systemic steroids decrease capillary permeability and stabilize the basement membrane. Tapered oral methylprednisolone (Medrol Dosepak) can be effective early. With regional intravenous (IV) blocks (methylprednisolone sodium succinate and lidocaine HCl), good results were reported in 20 of 24 patients when blocks were started within 6 months of symptoms. Overall, results are mixed.73 Corticosteroids have been used in open trials (n = 64-69)45 and in one controlled trial (n = 23)12 to treat CRPS I, all of limited methodologic quality. All the studies found corticosteroids to have a very pronounced beneficial effect. Corticosteroids may have a positive effect on CRPS I, but little is known as to the duration and dosage. Oral sympatholytics are poorly tolerated. Fatigue, sedation, hypotension, and sexual dysfunction are major problems.20 Three placebo-controlled studies have been carried out to date.26 One study (n = 20) involved administration of alendronate 3 days in a row.1 Another study evaluated the efficacy of clodronate (n = 32).77 In a third study, treatment comprised alendronate (40 mg: this dose is four times as high as that given for osteoporosis) administered to 40 CRPS I patients. In the three studies, the parameters in the group of patients treated with bisphosphonates improved significantly more than in the placebo group. Two studies of moderate quality and size investigated the effect of nifedipine and phenoxybenzamine in treating CRPS I.48,49,57 One retrospective study with 59 patients reports that nifedipine (20 mg per day) or phenoxybenzamine (up to 120 mg/day) are most effective for CRPS I in the acute phase.57 Both studies are primarily descriptive and the outcomes are subjective, failing to describe the nature of the improvement in patients’ conditions. There are indications that calcium channel blockers have some effect in the acute phase of CRPS I. Although they improve blood circulation, they also cause side effects, such as a drop in blood pressure and headache. Two placebo-controlled, randomized studies have been found that examined the use of gabapentin in neuropathic pain patients. The first study69 (n = 307) shows that gabapentin causes a modest but significant reduction in neuropathic pain symptoms 8 weeks after the start of treatment. It is unclear what this means for the CRPS I patients, who made up 28% of the sample population. In the second study (n = 58), a moderate effect on pain was found, but no significant reductions in other sensory abnormalities were found.75 Dizziness, sleepiness, and fatigue occurred significantly more often in patients taking gabapentin than in patients taking placebo. The effects of calcitonin have been evaluated in two meta-analyses and two systematic reviews. The meta-analysis carried out by Kingery et al41 reports conflicting findings as to the effects of calcitonin. In contrast, the meta-analysis carried out by Perez et al62 points to calcitonin having a positive effect on pain on average, and the review carried out by Forouzanfar et al26 also describes positive results for calcium-regulating drugs (including calcitonin) administered to CRPS I patients. There is conflicting evidence with respect to the efficacy of calcitonin for treatment of CRPS I. Lidoderm, a 5% lidocaine patch, is applied directly over the painful site. Systemic effects are unlikely, and the primary problem is application-site sensitivity. It is FDA approved for 12 hours on and 12 hours off. However, off-label use (increased time on) is common. Quality of life has been often shown to improve after 4 weeks of treatment (Fig. 14-16). One quasi-experimental study with seven patients evaluated the effects of lumbar and stellate blockades with lidocaine/bupivacaine compared with placebo (follow-up 2-2.5 weeks). No significant differences were found between active and placebo treatment with respect to initial peak pain reduction.65 An uncontrolled open study investigated the long-term effects (mean follow-up, 32 months; range, 7-48 months) of epidural administration of bupivacaine to 14 patients with CRPS I in the knee.15 Treatment was continued with continuous administration of a narcotic. No pain control data were described; however, 11 patients were seen to have a complete improvement of CRPS I symptoms at the end of the follow-up. Current literature suggests that some new treatments are just over the horizon. NMDA receptor–site blockers are being developed. Botulinum toxin might inhibit the release of glutamate. Pain and bone density can improve after intravenous administration of pamidronate, alendronate, or clodronate. Magnetic stimulation of the contralateral brain cortex can decrease pain.71 Inhalation of cannabinoids (marijuana), much touted in the news for their pain-relieving qualities, does not relieve neuropathic pain, and the side effects of somnolence, fatigue, hypotension, and memory disorder make them unlikely to improve CRPS symptoms.4 Dimethylsulphoxide (DMSO), a free-radical scavenger, has been tried in patients with CRPS I. A prospective crossover study30 with 20 patients found a positive effect of DMSO on the function of the affected limb. In addition, in 26 CRPS I patients, DMSO was found to be significantly more effective than the conventional regional ismelin block27 in reducing pain. A randomized double-blind trial conducted with 32 CRPS I patients86 also showed that five times daily use of topical DMSO cream provided significantly better results on CRPS I symptoms than placebo after 2 months of treatment. In general, DMSO generates lower (direct and indirect) costs than N-acetylcysteine (another available free-radical scavenger). Subgroup analysis indicates that N-acetylcysteine is more cost effective in patients with a cold form of CRPS I than DMSO. The opposite holds for warm forms of CRPS I.76 Robert J. Schwartzman, MD, a neurologist at Drexel University College of Medicine, is experimenting with a more radical approach. Cooperating with a group of anesthesiologists in Germany, he is treating patients who have refractory pain by injecting them with ketamine at doses high enough to induce coma for 5 days. Fifty percent improvement has been reported.32 The effects of a subanesthetic ketamine infusion (10 mg/hr up to 15-50 mg/hr) was assessed in a retrospective study of 33 patients with CRPS I or II.16 Twelve patients experienced a relapse and had a second course of infusions, 3 patients had a third course, by which pain disappeared completely in 83% of patients. The average duration of pain reduction (data of 20 patients) was 9.4 months. The side effects were intoxication, hallucinations, dizziness, nausea, light-headedness, and blurred vision. Recent investigations have suggested a role for vitamin C in the prevention of CRPS. In a double-blind, prospective, multicenter trial, 427 wrist fractures were randomly allocated to treatment with placebo or treatment with 200, 500, or 1500 mg of vitamin C daily for 50 days.85 The effect of gender, age, fracture type, and cast-related complaints on the occurrence of complex regional pain syndrome was analyzed. The prevalence of complex regional pain syndrome was 2.4% in the vitamin C group and 10.1% in the placebo group (P = .002). Early cast-related complaints predicted the development of CRPS, and based on these results, the authors recommended a daily dose of vitamin C 500 mg for 50 days to prevent CRPS. Additional investigations by the same author have yielded similar results,84 including those which suggest a benefit from vitamin C 2 days before surgery and for 50 days after surgery for trapeziometacarpal arthritis. Thirty four patients were administered 500 mg of vitamin C and followed for a minimum of 44 months. Contrary to another published report, which demonstrated a 13% CRPS rate with this procedure, there were no cases of CRPS in this study.84 A similar investigation regarding the role of vitamin C in the prevention of CRPS in patients undergoing foot and ankle surgery confirmed prior studies suggesting that vitamin C may be preventative.8 Implantable spinal electrodes for dorsal column stimulation to control intractable pain should be considered once a plateau in improvement with sympathetic blocks has been reached or in refractory cases. Based on the gate theory of pain control, a percutaneous route for implantation with low morbidity is now well developed. Long-term results are good, and late failures are primarily based on technical problems. Permanent placement should be preceded by a trial with a temporary electrode.60 There are numerous studies investigating these devices. Patients with chronic refractory CRPS I were randomly allocated to spinal cord stimulation (SCS) plus physiotherapy or physiotherapy alone. Trial stimulation proved successful in 24 of the 36 patients; only these patients underwent a procedure to implant a permanent SCS device. Pain intensity was reduced by 2.4 cm on a visual analogue scale after 6 months in the group receiving spinal cord stimulation plus physiotherapy compared with a group receiving only physiotherapy.37 At 2 years follow-up, pain decrease in the SCS group was 2.1 cm more than pain decrease observed in the physiotherapy group.39 Quality of life improved only in the patients with an implanted system; function remained unchanged. Two retrospective cohort studies have investigated effects of SCS on pain relief (n = 23-31).11,38 All studies relate to carefully selected patients with refractory CRPS I. There is no scientific evidence for SCS being effective in acute CRPS I. Complications requiring further surgery do occur in 25% to 50% of patients.74 The efficacy of surgical sympathectomy was addressed in a systematic review,50 based on analysis of retrospective cohort, including 7 to 73 individuals. All the studies report a clear reduction in pain resulting from sympathectomy, whereby the extent of pain relief declines over time. Long-term follow-up studies (>1 year) indicate that the chance of success is greatest if treatment is given within 3 months after the initial trauma.68 Recurrence of SMP can occur secondary to upregulation of distal receptors, producing increased sensitivity to catecholamines. In some cases, postsympathectomy neuralgia in the thigh can be distressing. Sympathectomy for multiple extremities leads to problems with thermal regulation and with bowel or bladder control.29 It is important that the orthopaedic surgeon maintain constant vigilance for the possibility of an underlying neurologic lesion (e.g., neuroma, tarsal tunnel syndrome) associated with CRPS II. Maximum control of the secondary hyperalgesia must be obtained before treating the peripheral nerve disease.2,6,21,78 If surgery is planned in an extremity previously involved in CRPS, conductive (spinal) anesthesia should be used. General anesthesia is associated with higher recurrence rates. At times, despite adequate planning, surgery for fixed deformity fails or symptoms recur. In this case, carefully planned bracing is often successful (Fig. 14-17).
Soft Tissue Disorders of the Foot
Complex Regional Pain Syndrome (Reflex Sympathetic Dystrophy)
Physiology
Complex Regional Pain Syndrome Type I
Complex Regional Pain Syndrome Type II
Psychiatric Manifestations
Incidence
Differential Diagnosis
 Malingering, worker’s compensation
Malingering, worker’s compensation
 Medicolegal status of the condition
Medicolegal status of the condition
 Personal family or sexual conflict
Personal family or sexual conflict
Physical Examination
Diagnostic Evaluation
Symptom
Technique
Dynamic mechanical allodynia
Stroking with brush, gauze, or cotton applicator; hair movement
Vibration by tuning fork
Static mechanical allodynia
Mechanical light pressure
Mechanical hyperalgesia
Manual pinprick by safety pin
Calibrated stimulator
Mechanical summation
Apply dynamic stimuli every 2-3 sec, 3-6 times
Heat allodynia
Contact with objects kept immersed in 40°-42° C (104°-107.6° F) water
Heat hyperalgesia
Contact with objects kept immersed in 45°-47° C (113°-116.6° F) water (feels weakly painful to the clinician)
Cold allodynia
Contact with room-temperature metal object, with refrigerated object, and with coolants
Conservative Treatment
Physical Therapy
Nonpharmacologic Options
Transcutaneous Nerve Stimulation
Acupuncture
Nerve Blocks
Pharmacologic Agents*
Opioids
 Impaired control over drug use
Impaired control over drug use
 Refusal to participate in a medication taper
Refusal to participate in a medication taper
 Reports that nothing but a specific opioid works
Reports that nothing but a specific opioid works
 Preference for short-acting versus long-acting opioids
Preference for short-acting versus long-acting opioids
 Use of multiple prescribers and pharmacies
Use of multiple prescribers and pharmacies
 Not taking medicine as prescribed
Not taking medicine as prescribed
 Loss of medication more than once
Loss of medication more than once
 Use of nonprescribed psychoactive drugs in addition to prescribed medication
Use of nonprescribed psychoactive drugs in addition to prescribed medication
Atypical Opioids
Paracetamol
Nonsteroidal Antiinflammatory Drugs (NSAIDs)
Antidepressants
Systemic Steroids
Oral Sympatholytics
Bisphosphonates
Calcium Channel Blockers
Anticonvulsants
Calcitonin
Topical Treatments
Local Anesthetics
New Treatments
Free-Radical Scavengers
Vitamin C
Pain Therapies
Surgical Treatment
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Soft Tissue Disorders of the Foot