Slipped Capital Femoral Epiphysis
Robert M. Kay
Young-Jo Kim
Slipped capital femoral epiphysis (SCFE) is defined as the displacement of the femoral head relative to the femoral neck and shaft. The term slipped capital femoral epiphysis is actually a misnomer. The femoral head is stabilized in the acetabulum, whereas the femoral neck and shaft move relative to the femoral head and acetabulum. In almost all cases of SCFE, the proximal femoral neck and shaft move anteriorly and rotate externally relative to the femoral head (1). If progression occurs to the point at which the femoral neck is completely anterior to the femoral head, then proximal migration of the femoral neck occurs as well.
EPIDEMIOLOGY
The epidemiology of SCFE has been reported frequently in the last century. The male population with SCFE outnumbers the female population by 1.4 to 2.0 in most studies (2, 3, 4, 5, 6, 7, 8, 9 and 10). The annual incidence is 2 to 13 per 100,000, and the cumulative risk is between 1 per 1000 and 1 per 2000 for the male population and is between 1 per 2000 and 1 per 3000 for the female population (11, 12, 13 and 14). Incidence of SCFE varies significantly among different populations, with higher incidences in those groups with higher mean body weights (15). Loder (15) has noted more than a 40-fold difference in the incidence among differing races, with the highest rate being found in Polynesian children and the lowest rate being found in children from the Indo-Mediterranean region. In a more recent survey of the epidemiology of SCFE, Lehmann et al. (16) reported that SCFE remains more common in boys (13/100,000) than girls (8/100,000) but that that the age of onset appears to be getting younger. In the same study, the incidence was almost 4 and 2.5 times the incidence in blacks and Hispanics compared to whites (16).
Most children with SCFE are peripubertal. Loder (15) reported an average age of 12 ± 1.5 years for girls and 13.5 ± 1.7 years for boys in an international study carried out with more than 1600 patients. At the time of presentation, approximately 80% of the boys are reported to be between 12 and 15 years and 80% of the girls between 10 and 13 years (17). Onset of SCFE is unusual for children of either sex <10 years old and for girls older than 14 and boys older than 16. Diagnosis of SCFE in such patients should raise the orthopaedist’s suspicion that an underlying metabolic or systemic condition may have played a causative role. Furthermore, subclinical endocrine abnormalities may be common. In a prospective study, 14 patients with SCFE were screened for 154 endocrine abnormalities. Despite lack of clinical symptoms, 27% of the surveyed laboratory findings had some evidence of abnormality (18).
The range of skeletal ages of children with SCFE has been reported to be significantly narrower than the range of their chronologic age (10, 19, 20). Most of the children with SCFE have open triradiate cartilage and are Risser 1 (21).
Obesity has been reported in 51% to 77% of patients with SCFE (6, 15, 22, 23 and 24). Approximately 50% of the patients are at or above the 90th percentile for weight (25), and approximately 70% are above the 80th percentile (26). Obese children with slow maturation appear to be at especially high risk for SCFE (27).
Unilateral involvement is noted in 80% of children with SCFE at the time of presentation, with left hip involvement
in most unilateral cases (12, 13, 15, 28, 29). In addition to the 20% who initially present with bilateral SCFE, 10% to 20% develop a symptomatic contralateral slip in adolescence (6, 13, 30, 31 and 32). Long-term studies have reported radiographic evidence of a long-term bilateral involvement in as many as 80% of the patients (33), although most series report bilateral involvement at long-term follow-up in the 60% range in adulthood (13, 31). Attempts have been made to identify demographic and radiographic factors associated with the development of bilateral SCFE. However, the data remain controversial. Among the factors associated with increased risk include young chronologic age (girls <12 years and boys <14 years), open triradiate cartilage, and body mass index >35 (34, 35 and 36).
in most unilateral cases (12, 13, 15, 28, 29). In addition to the 20% who initially present with bilateral SCFE, 10% to 20% develop a symptomatic contralateral slip in adolescence (6, 13, 30, 31 and 32). Long-term studies have reported radiographic evidence of a long-term bilateral involvement in as many as 80% of the patients (33), although most series report bilateral involvement at long-term follow-up in the 60% range in adulthood (13, 31). Attempts have been made to identify demographic and radiographic factors associated with the development of bilateral SCFE. However, the data remain controversial. Among the factors associated with increased risk include young chronologic age (girls <12 years and boys <14 years), open triradiate cartilage, and body mass index >35 (34, 35 and 36).
Some authors have noted significant seasonal variation in the incidence of SCFE at latitudes above 40 degrees, but not in lower latitudes (37, 38 and 39). Others have not noted any seasonal variation (13). Such data appear to have little impact on the diagnosis and treatment of children with SCFE.
In summary, SCFE is most commonly seen in overweight, peripubertal children. Although any child presenting with hip, groin, thigh, or knee pain must be evaluated for possible hip pathology, the orthopaedist should be particularly suspicious of the possibility of SCFE in overweight, peripubertal children.
ETIOLOGY
In most children with SCFE, the precise etiology is unknown. Regardless of the underlying etiology, the final common pathway appears to be a mechanical insufficiency of the proximal femoral physis to resist the load across it (40). SCFE may be thought of as occurring because of physiologic loads across an abnormally weak physis or abnormally high loads across a normal physis.
Conditions that weaken the physis include endocrine abnormalities, systemic diseases (such as renal osteodystrophy), and previous radiation therapy in the region of the proximal femur (41, 42, 43, 44, 45 and 46). Multiple mechanical factors have been postulated to account for abnormally high loads across the proximal femoral physis in children with SCFE, including obesity and anatomic variations in the proximal femoral and acetabular morphology.
Endocrine Factors.
The endocrinologic basis of SCFE has been studied both in vivo and in vitro. For more than 50 years, laboratory studies have demonstrated that estrogen strengthens and testosterone weakens the physis (47, 48 and 49). These effects appear to be secondary to the impact that these hormones have on physeal width since mechanical strength of the physis varies inversely with physeal width (47, 49, 50).
Endocrinopathies appear to account for 5% to 8% of the SCFE cases, and SCFE has been estimated to be six times more common in patients who have an endocrinopathy than in those who do not (41, 42, 43, 44, 45 and 46, 51, 52, 53, 54, 55, 56, 57 and 58). Although one recent study showed frequent endocrine abnormalities, most investigators have been unable to demonstrate consistent abnormalities in most children with SCFE (25, 26, 59, 60).
The most common endocrinopathies in children with SCFE are hypothyroidism, panhypopituitarism, growth hormone (GH) abnormalities, and hypogonadism (41, 42, 43, 44, 45 and 46, 51, 52, 53, 54, 55, 56, 57 and 58). Other endocrine causes of SCFE include hyperparathyroidism or hypoparathyroidism (41, 44, 61). The increased prevalence of hypothyroidism in children with Down syndrome is a likely explanation for the increased risk of SCFE in these children (62, 63 and 64).
The relative risk of SCFE is increased in children with GH deficiency, both prior to and during GH treatment (65, 66 and 67). Other children with short stature and normal GH levels do not appear to share the same increased risk of SCFE (65, 66). The initial diagnosis of hypothyroidism is often made after the diagnosis of SCFE; in most children with SCFE and GH deficiency, the endocrine abnormality is known prior to the diagnosis of SCFE (41).
SCFE has been noted to be most common in children around the time of puberty. It may be that the abnormalities in the complex interplay of hormones at puberty put their hips at risk for SCFE (26, 68). Laboratory studies in rats have also shown a decreased physeal strength at puberty (69).
Because the rate of endocrinopathy in children with SCFE is relatively low, previous authors have recommended against the routine screening of patients with SCFE without clinical evidence of an endocrinopathy (59). Burrow et al. (52) reported that a person’s height below the 10th percentile was the only useful screening characteristic for endocrine abnormalities; the sensitivity and the negative predictive value of using height below the 10th percentile as a cutoff were each reported to be at least 90%.
On the basis of the aforementioned data, routine screening of all patients with SCFE for any potential endocrine disease is not warranted. For children with suspected endocrine disease (including those who are younger than 10 years or older than 15 years and those who are of short stature), thyroid function tests should be carried out. GH levels should be checked for children of short stature. It is important to remember that most children with SCFE and thyroid dysfunction have no known history of any thyroid dysfunction at the time of presentation with SCFE. Among other children with both endocrinopathies and SCFE, the underlying endocrine disorder is often known prior to the diagnosis of SCFE.
Other Systemic Diseases.
Previous radiation therapy to the region of the femoral head also increases the risk of SCFE (70, 71). The absolute risk of SCFE in patients with previous radiation therapy is unknown, although a risk as high as 10% has been cited (70). Unlike the typical patient with SCFE, children with SCFE following previous radiation therapy have been reported to have a median weight at the 10th percentile (71).
Renal osteodystrophy is associated with a sixfold to eightfold increased risk of SCFE (66). The incidence of SCFE has been reported as 0.03 to 0.64 per 1000 person-years among patients with end-stage renal disease receiving GH, with the highest rates in those patients who were on dialysis and receiving GH (72). Patients with renal osteodystrophy and SCFE are noted to be small in both weight and height (73).
The increased rate of SCFE associated with renal osteodystrophy is due to secondary hyperparathyroidism in these children, and medical management of the secondary hyperparathyroidism is of primary importance (73). If the hyperparathyroidism is controlled, slip progression will become rare, and surgical stabilization may not be necessary (73). Unlike the situation in other causes of SCFE, the displacement in patients with renal osteodystrophy is often through the metaphysis (35% of reported SCFE in one series), and other epiphyses have also been known to displace (73, 74 and 75). Bilateral involvement has been reported in 82% to 95% of the patients with SCFE and renal osteodystrophy in large series studies (73, 75). That many of these so-called SCFE cases do not occur through the physis may partly be the reason for the poorer results in the treatment of SCFE in children with renal osteodystrophy.
Immunology.
Elevated levels of serum immunoglobulins and the C3 component of complement have previously been reported in patients with SCFE (76). In patients with chondrolysis, serum immunoglobulin M level was elevated as well (76). More recent studies have failed to show such abnormalities in serum levels, although synovial fluid abnormalities were noted in patients with SCFE (77, 78). One study reported that plasma cells were a significant component of the synovitis in SCFE (77). In the same study, two of three patients with IgG and C3 present on synovial immunofluorescence developed chondrolysis (77). A later study revealed the presence of immune complexes in the synovial fluid in 10 of the 11 hips with SCFE (91%), but not in 2 of the 21 joints without SCFE (10%) (77, 78). The role of these immune complexes in SCFE has not been defined.
Genetics.
A genetic basis for SCFE has not been definitively established. Among the patients with SCFE, a second member of their family has been reported to be affected in 3% to 7% of the cases in most series of studies carried out (10, 24, 32, 79, 80, 81, 82, 83, 84, 85 and 86). SCFE has been reported in identical twins (79, 81, 87) and has been found to have autosomal dominant inheritance with variable penetrance in familial cases (85, 86). Whether this is due simply to a genetic predisposition for SCFE or due also to a tendency toward other risk features (such as obesity) remains unclear (85, 88).
Mechanical Factors.
A variety of mechanical factors appear to play a role in the etiology of SCFE. Anatomic risk factors in the proximal femoral and acetabular morphology have been described. The high incidence of obesity in this patient population also suggests a mechanical role in the etiology of SCFE.
An association of SCFE with a decreased femoral anteversion has been reported, and this has been attributed to increased shear force across the proximal femoral physis in such patients (93, 94). Anteversion values of the unaffected hips in the same patients were closer to normal (93).
Finally, reduced femoral anteversion has been noted in obese adolescents compared to adolescents of normal weight (95). This relative retroversion in obese adolescents may help explain the increased incidence of SCFE in this population group.
Decreased femoral neck-shaft angle in the hips of patients with SCFE compared to the hips of unaffected persons has also been reported (94). Such a decrease in the neck-shaft angle results in a more vertical physis, which may increase the shear force across the physis. Proximal femoral physeal inclination has previously been shown to change significantly between the ages of 9 and 12 years in humans, which is a potential contributing factor for SCFE (96). In the laboratory, the shear strength has also been shown to vary with physeal inclination (50).
Children with deeper acetabuli appear to be at greater risk for SCFE (97). The supposition is that with the capital femoral epiphysis anchored more deeply in the acetabulum, forces across the physis may be exaggerated, especially at the extremes of the range of motion. Variability in acetabular depth has been suggested as a potential cause for differences in the incidence of SCFE among different races. A recent study of acetabular morphology in patients with trauma calls this finding into question (98). It is possible that this study did not find such a correlation either because of limited sample size and/or because SCFE may simply be occurring in a small subset of the population who are outliers regarding such measures as acetabular depth.
Kordelle et al. (99) have not found any difference in acetabular morphology in the affected and the unaffected hips of children with SCFE. The lack of such acetabular differences is likely because SCFE generally occurs at an age at which little potential remains for acetabular remodeling, and this may help explain the high incidence of bilateral SCFE. Such bilateral acetabular symmetry in those with unilateral SCFE suggests that even if increased acetabular depth is a risk factor, there must be other etiologic factors involved as well.
Chung et al. (100) reported that the mechanical forces across the femoral head during gait can be 6.5 times body weight and that such forces may be enough to cause a SCFE in an obese patient with a normal physis. A finite element analysis by Fishkin et al. (101) showed that, in an “overweight” child, the combination of proximal femoral varus and retroversion could result in sufficient forces at the physis to cause SCFE. Other authors have confirmed that mechanical forces across the hip during normal activities such as running are great enough to potentially cause SCFE (102).
In summary, the etiology of SCFE appears to be complex and is likely to be multifactorial. Endocrinopathies, other systemic diseases, and local abnormalities (such as those caused by previous radiation exposure) have been noted to result in an increased risk of SCFE. Studies carried out on humans and animals indicate that such an increased risk of SCFE appears related to the impact that these maladies have on the strength of the growth plate. The association of hypothyroidism in children with Down syndrome and of secondary
hyperparathyroidism in those with renal osteodystrophy explains the sometimes unclear risk profile of SCFE in certain groups of patients. Subtle abnormalities of hormonal balance at the time of puberty may also be partially responsible for SCFE in children without any definite systemic or hormonal abnormalities.
hyperparathyroidism in those with renal osteodystrophy explains the sometimes unclear risk profile of SCFE in certain groups of patients. Subtle abnormalities of hormonal balance at the time of puberty may also be partially responsible for SCFE in children without any definite systemic or hormonal abnormalities.
Mechanical factors also appear to play an etiologic role in the development of SCFE. Clearly, systemic and local factors alone cannot explain all the cases of SCFE because many patients with the aforementioned abnormalities do not develop a SCFE. In addition, most patients with SCFE provide evidence of increased forces across the proximal femoral physis due to one or more potential causes, including obesity and variations in the proximal femoral and/or acetabular morphology.
CLINICAL FEATURES
Traditionally, classification of SCFE has been made on a temporal basis. Chronic slips are those causing symptoms for a period of at least 3 weeks, whereas acute slips are those that are symptomatic for <3 weeks. Acute-on-chronic slips are those with an acute exacerbation of the symptoms following a prodrome of symptoms of at least 3 weeks’ duration. Chronic slips appear to account for 80% to 90% of all SCFE. Although not part of the preceding scheme, a “preslip” has been defined as a symptomatic hip with evidence of physiolysis prior to true movement of the femoral neck relative to the femoral head.
In 1993, Loder et al. (103) suggested a new classification of SCFE based on physeal stability. An unstable SCFE was defined as occurring in an extremity upon which the child had such severe pain that walking is not possible even with crutches. With a stable slip, the child can walk with or without crutches. Unstable SCFE account for 50% to 60% of acute SCFE and for 5% to 10% of all SCFE (103, 104, 105 and 106). This classification of SCFE based on stability has largely supplanted the aforementioned temporal classification scheme because of its improved ability to predict both osteonecrosis (ON) and poorer outcomes. Whereas ON is usually reported in 10% to 15% of acute SCFE, Loder et al. (103) reported ON in 47% of unstable SCFE and 0% stable SCFE in their landmark paper. Even in cases of acute SCFE, only the unstable subset appear to be at significant risk for ON and a poor outcome (103, 107).
The most common findings at presentation of SCFE include pain, limp, and decreased range of motion of the hip. Hip or groin pain in an obese, peripubertal child is highly suggestive of SCFE. However, hip pain is absent in as many as 50% of the children with SCFE, including up to 8% with a painless limp (108). Pain is localized to the knee and/or distal thigh in 23% to 46% of cases (4, 6, 108, 109). Previous studies have noted that distal thigh and/or knee pain often result in significant misdiagnosis of SCFE, delay in diagnosis, unnecessary radiographs, increased slip severity, and sometimes in unnecessary knee arthroscopy (4, 6, 23, 108, 109, 110 and 111). These findings indicate the importance of examining the hip in all children presenting with distal thigh and/or knee pain.
Symptoms of SCFE are generally present for weeks to several months prior to presentation to the orthopaedist (15, 112). Although patients report a specific inciting event as the cause of pain in approximately 50% of cases, severe trauma is rarely reported (108). Even when trauma is reported, further questioning often reveals a history of pain for weeks or months preceding the inciting event.
A significant proportion of the 5% to 10% of children with unstable SCFE present with an acute onset of severe hip pain in the absence of prodromal symptoms (15, 113, 114). Such SCFE often follow mild trauma.
As has been noted, most children with SCFE are obese. Short stature (height less than the 10th percentile) has been reported to be an indicator of increased risk for underlying systemic disease in children with SCFE (52). Loder and Greenfield (115) noted that SCFE due to an underlying cause (such as underlying systemic disease or previous radiation exposure) was much greater in children older than 16 years and/or those who were below the 50th percentile for weight at the time of presentation.
When a child presents with hip, groin, thigh, or knee pain, care must be taken to evaluate both hips. The physician needs to be persistent when asking about symptoms in both hips, because a child often initially complains of only the more symptomatic hip in cases of bilateral SCFE.
One of the most helpful tip-offs in these patients is the observational gait analysis when the child walks into the examining room. The limp in children with SCFE is due to several gait deviations. Hip abductor weakness commonly manifests as a trunk lean to the affected limb in stance (Trendelenburg gait). If there is marked pain, an antalgic gait (decreased stance phase on the affected limb) will be present as well. Finally, because of the external rotation of the femoral neck and shaft (relative to the femoral head), the foot and knee progression angles on the affected side are often markedly external. Children with unilateral involvement have significant asymmetry of foot and knee progression angles with a unilateral Trendelenburg gait, whereas children with bilateral SCFE present with a more “waddling” gait bilaterally, and bilateral external foot and knee progression.
On physical examination, range of motion of the hips— including the rotational profile of the hips—should be measured and compared. Hip flexion to 90 degrees is unusual, and hip flexion contractures are common. Because both hip flexion and extension are lost, there is significant diminution of the sagittal arc. Hip abduction is significantly limited both actively and passively, and the hip abductors are weak.
Hip rotation is abnormal because of both the abnormal anatomy and the synovitis that accompany SCFE. Loss of the hip internal rotation is combined with preservation of (or even an increase in) external rotation. With a SCFE, the hip will
automatically fall into external rotation (the so-called obligate external rotation) as it is progressively flexed. Obligate external rotation of the hip(s) is essentially pathognomonic for SCFE. In cases of unilateral SCFE, comparison with the rotation of the contralateral hip clearly demonstrates this change in the arc of motion. In bilateral SCFE, both hips will demonstrate this shift toward external rotation.
automatically fall into external rotation (the so-called obligate external rotation) as it is progressively flexed. Obligate external rotation of the hip(s) is essentially pathognomonic for SCFE. In cases of unilateral SCFE, comparison with the rotation of the contralateral hip clearly demonstrates this change in the arc of motion. In bilateral SCFE, both hips will demonstrate this shift toward external rotation.
In summary, any patient between the ages of 10 and 16 years who presents with a limp and pain in the groin, hip, thigh, or knee should be considered to have a SCFE until proven otherwise. Diagnoses such as pulled groin muscles are rarely correct in children, although such misdiagnoses are commonly made in children with SCFE. The index of suspicion for the diagnosis of SCFE is markedly increased in obese, peripubertal children with a limp, external foot progression, and pain in the groin, hip, thigh, or knee. The index of suspicion is also very high in patients with a known history of endocrine abnormalities and in those with underlying diseases associated with endocrine abnormalities, such as Down syndrome and renal osteodystrophy.
RADIOGRAPHIC FEATURES
Radiographs.
High-quality anteroposterior and lateral radiographs of each hip should be obtained to confirm the diagnosis of SCFE. Because of the high frequency of bilateral SCFE, bilateral imaging has been recommended for decades (23, 116, 117). In an unstable, acute SCFE, a frog lateral view is not obtained preoperatively in order to avoid causing pain and because of the potential for displacement of the SCFE. However, it is usually possible to obtain a crosstable lateral view of the affected hip without adversely affecting the unstable SCFE.
On the anteroposterior view, widening and irregularity of the physis may be the only radiographic findings prior to, or with minimal, displacement of the femoral neck and shaft relative to the femoral head. Cowell (108) noted that the displacement may not be evident in 14% of the anteroposterior views. Another common finding on the anteroposterior view is a decreased height of the capital femoral epiphysis when the epiphysis lies posterior to the femoral neck. As slipping progresses, the metaphysis appears progressively more lateral relative to the acetabular teardrop, and an increased radiodensity of the proximal metaphysis (the so-called metaphyseal blanch) may be noted (118). Osteopenia of the affected hip is common as well.
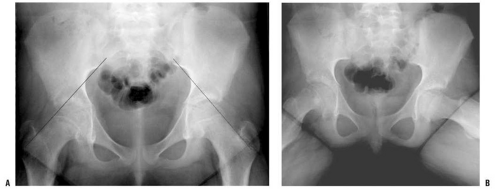
Lateral views are more sensitive for detecting mild degrees of slip. With increased magnitude of slipping, the SCFE becomes evident on the anteroposterior view as well. Normally, a portion of the femoral head lies lateral to Klein line (a line drawn along the lateral border of the femoral neck) (116) (Fig. 25-1A,B). A SCFE is present if the Klein line lies cephalad to the femoral head, or if the amount of femoral head cephalad to the Klein line is less than that is seen for the contralateral hip.
Crosstable lateral views are often cited as more reliable than frog lateral views in the assessment of SCFE, which may be due to difficulties with the positioning of these children (119, 120). However, using a femoral model, Loder (121) reported that an accurate representation of the SCFE was obtained with either crosstable or frog lateral views when the
femur is rotated externally by 30 degrees or less. The value of other specialized views, such as the Billing lateral, is still being debated (121, 122).
femur is rotated externally by 30 degrees or less. The value of other specialized views, such as the Billing lateral, is still being debated (121, 122).
The degree of slip is commonly quantified as the amount of femoral head displacement as a percentage of the femoral neck diameter, and was first described by Wilson in 1938 (23). Slips have been categorized as mild (<33%), moderate (33% to 50%), and severe (more than 50%) (6, 24). Although frequently used, this measurement can be inconsistent because of variations in patient positioning and can change over the passage of time because of proximal femoral remodeling. This measurement should therefore be used only in the evaluation of SCFE prior to remodeling (123).
Southwick (124) recommended measuring the angles between the proximal femoral physis and the femoral shaft, the so-called head-shaft angles, on both anteroposterior and lateral radiographs. The difference between these two angles obtained at the affected and the unaffected sides determines the degree of abnormal alignment and is often referred to as Southwick angles. The lateral view gives an indication of posterior angulation. A difference of <30 degrees has been deemed mild, a difference of 30 to 50 degrees moderate, and more than 50 degrees is deemed as severe (125).
The angle between the proximal femoral physis and femoral neck, the so-called head-neck angle, may be measured but is less reliable because remodeling adjacent to the SCFE may artificially decrease this number in the absence of clinically significant changes in femoral version.
Other Imaging Studies.
Radiographs are sufficient for the evaluation of most children with SCFE. However, additional imaging may be warranted in special circumstances, such as in the evaluation of a presumed “preslip” in a child with normal radiographs, or in the early evaluation of a patient with SCFE at risk for ON.
Computed tomography (CT) scans are rarely needed as a part of the initial assessment of children with SCFE (120). Some authors report that CT scan is more accurate than radiographs in evaluating the anatomy of SCFE (120), whereas others report comparable reliability between the two modalities (126, 127). If a child presents very late in the course of SCFE, a CT scan may be useful in determining whether sufficient physeal closure has already occurred, thereby potentially precluding the need for an in situ fixation. A CT scan may also be helpful postoperatively in determining whether any hardware used during surgery has accidentally penetrated the joint surface. This is particularly true in the case of femoral head collapse in association with ON of the femoral head.
Ultrasound has been championed by some authors, but currently appears to have little use in the routine evaluation of patients with SCFE (128, 129, 130 and 131). Previous studies using ultrasound images have indicated the presence of effusion in 42% to 60% of patients with SCFE (130, 131). In experienced hands, ultrasound may have a role in confirming a suspected case of SCFE in the absence of any radiographic findings, but magnetic resonance imaging (MRI) is more commonly used in such situations.
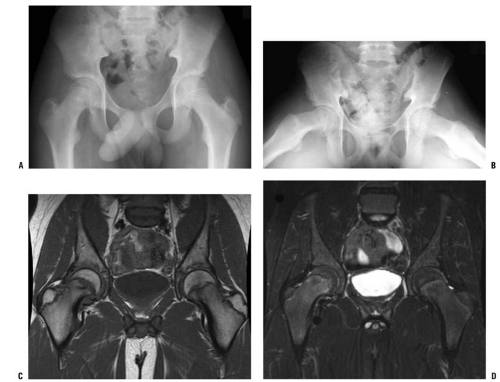
MRI often plays an important role in the evaluation of hips of patients who are presumed to have SCFE but have normal radiographs, and MRI may also be used for the early detection of ON. The MRI findings in SCFE have been well described (127, 132, 133 and 134). Physeal widening, osseous edema adjacent to the physis, and the anatomic deformity associated with SCFE are typically seen, with the findings of physeal widening and irregularity as well as osseous edema adjacent to the physis seen in cases of “preslips” (134). In a child with suspected SCFE and normal radiographs, MRI is useful in determining whether a preslip is present (Fig. 25-2). Currently, MRI scanning is rarely used in evaluating patients with evident SCFE.
MRI may be used to assess femoral head circulation in order to evaluate for the presence of ON, as well as its extent and distribution if present. Unfortunately, metal artifact may significantly interfere with MRI signals. The findings of ON seen on MRI scans have not been correlated with subsequent radiographic findings and the clinical course of the affected hips.
Bone scans may be used to assess femoral head viability in potential cases of ON of the femoral head, with decreased uptake being evident in cases of ON. Multiple studies have reported the utility of bone scanning in the detection of ON in SCFE (130, 135, 136). Sensitivity in detecting ON has been 100% in several series, although a false-negative bone scan has been reported in a child who went on to develop mild ON (130, 135, 136 and 137).
Although pretreatment bone scans are quite sensitive, they are also associated with false-positive results (i.e., an abnormal bone scan in a hip that does not develop ON). In two series, false-positive bone scans have been reported in one of the six (17%) (136) and two of three (67%) hips that were imaged (130).
Since pretreatment bone scans and MRI do not generally change treatment, they are not routinely obtained in children with SCFE at most centers.
PATHOANATOMY
Because the femoral head is relatively fixed inside the acetabulum, the slip is best thought of as a slip of the proximal femoral neck and shaft relative to the femoral head. In children younger than 3 years, the perichondral ring imparts significant physeal stability, whereas the mammillary processes of the physis are primarily responsible for increasing physeal shear strength thereafter (100).
In laboratory rats, physeal cracks are evident in the planes of shear stress used to create SCFE (69). The mechanical patterns of physeal fracture and the zone through which physeal shear causes fractures have been shown in rabbits to vary with increasing age and with the direction of loading (138, 139).
In humans, the direction of slip has been known for decades (1). In most cases, the proximal femoral neck and shaft migrate anteriorly and rotate externally, although slips have been noted to occur in other directions (140, 141). Previous authors have confirmed this anatomy and suggested a torsional force as the cause of acute SCFE (142). With progression of the slip, the femoral neck may come to lie completely anterior to the femoral head. When this occurs, proximal migration of the proximal femur is possible (Fig. 25-3). However, most SCFE do not appear to progress to this point, and the apparent varus seen radiographically has been attributed to parallax (143, 144). Degenerative changes, including cyst formation, may be seen in the anterior femoral neck and/or acetabulum because of impingement of the anterior femoral neck against the acetabulum during hip flexion, and such changes may be evident within years of the diagnosis of SCFE.
On the basis of computer modeling, Rab has noted that metaphyseal impingement limits the motion in severe SCFE
(145). He reported that as the slip angle increases, progressively greater external hip rotation is necessary to avoid anterior impingement of the proximal femoral metaphysis against the acetabulum during gait. Such levering can damage the anterosuperior acetabular cartilage and/or cause posterolateral labral injuries (145, 146, 147 and 148). Intraoperative evaluation by other authors has confirmed the mechanical impingement of the metaphysis against the superomedial acetabulum, with resulting cartilage and labral damage (149). Femoroacetabular impingement has been suggested as a cause of idiopathic arthritis as well (150). As noted by Rab (145), as the proximal femur remodels and motion returns toward normal, an increasing portion of the remodeled metaphysis becomes an intra-articular weight-bearing surface, potentially contributing to late osteoarthritis (OA).
(145). He reported that as the slip angle increases, progressively greater external hip rotation is necessary to avoid anterior impingement of the proximal femoral metaphysis against the acetabulum during gait. Such levering can damage the anterosuperior acetabular cartilage and/or cause posterolateral labral injuries (145, 146, 147 and 148). Intraoperative evaluation by other authors has confirmed the mechanical impingement of the metaphysis against the superomedial acetabulum, with resulting cartilage and labral damage (149). Femoroacetabular impingement has been suggested as a cause of idiopathic arthritis as well (150). As noted by Rab (145), as the proximal femur remodels and motion returns toward normal, an increasing portion of the remodeled metaphysis becomes an intra-articular weight-bearing surface, potentially contributing to late osteoarthritis (OA).
Multiple studies have investigated the pathologic changes in SCFE (138, 139, 151, 152, 153, 154, 155, 156, 157, 158 and 159). Multiple authors have noted the replacement of normal physis with abnormal cartilage, fibrocartilage, and fibrous tissue (156, 159). The physis is often hypocellular, with increased amounts of ground substance in lieu of the normal columnar architecture (151, 157). Others have noted a widening of the physis, with a loss of normal organization and the presence of clefts within the physis (158). Subsequent authors have confirmed the columnar disorganization with cartilage cell clumping in the physis, metaphysis, and epiphysis (157, 159). Groups of cartilage cells have been noted between metaphyseal trabeculae (155, 157, 159). Collagen fibrils are markedly diminished in the hypertrophic zone (157). The resting zone is essentially normal (155, 157). The proliferative zone has less densely packed collagen and increased disorganization, with ground substance replacing the normal chondrocytes. The hypertrophic zone is much larger than usual (up to 80% of the physeal width in comparison to 15% to 30% in normal physes) with marked disorganization, increased ground substance, and significant staining for glycoproteins (155, 157). Cell degeneration and death have been noted in the proliferative and hypertrophic zones (151, 152 and 153). The slip occurs through the proliferative and hypertrophic zones of the physis in an irregular pattern (68, 155, 157, 159). Histologic sections of the physis in SCFE before and after in situ fixation demonstrate a return to a more normal architecture following fixation; such findings have been postulated to indicate that mechanical stabilization of the physis, with removal of the abnormal shear forces across the physis, allows at least a partial reversal of the pathology seen with SCFE (154).
BLOOD SUPPLY
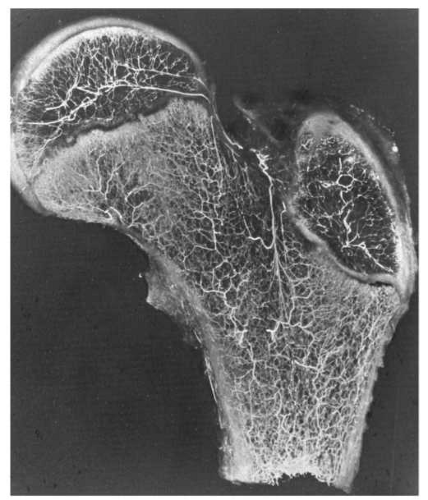
ON is one of the few potentially devastating complications associated with SCFE, and understanding the proximal femoral blood supply is important in attempting to minimize the frequency of this complication. The blood supply of the proximal femur can be divided into the intraosseous and extraosseous components, as has been well documented by Crock and subsequently by Chung (160, 161) (Fig. 25-4). Chung (160) noted that these components are present in an individual at birth and persist without significant change into adulthood. In cases of SCFE, the blood supply can be disrupted because of the SCFE itself (especially in cases with unstable SCFE), and it may also be compromised at the time of surgery.
It appears that the cause of ON is likely to be the disruption of the blood supply, which may occur because of displacement at the time of injury or at any time prior to operative fixation. Angiography performed in 12 patients with SCFE preoperatively showed filling of the superior retinacular artery in all 7 stable slips and in only 2 of the 5 unstable slips (162). In one of the three unstable SCFE without preoperative filling of the superior retinacular artery, postoperative angiography demonstrated appropriate filling (162).
Extraosseous Blood Supply.
The extraosseous blood supply to the proximal femur may be disrupted in acute SCFE and has been well described. An arterial ring at the base of the femoral neck gives rise to ascending cervical arteries that penetrate the hip capsule and provide circulation to the femoral head, neck, and greater trochanter (160, 161). The
arterial ring at the base of the femoral neck consists of the lateral femoral circumflex artery, which runs anteriorly and constitutes the anterior portion of the arterial ring, and the medial femoral circumflex artery, which travels posteriorly and constitutes the medial, lateral, and posterior portions of the ring. The ring is most commonly incomplete, without communication between the branches from the medial and lateral circumflex arteries.
arterial ring at the base of the femoral neck consists of the lateral femoral circumflex artery, which runs anteriorly and constitutes the anterior portion of the arterial ring, and the medial femoral circumflex artery, which travels posteriorly and constitutes the medial, lateral, and posterior portions of the ring. The ring is most commonly incomplete, without communication between the branches from the medial and lateral circumflex arteries.
Ascending cervical arteries (also known as retinacular vessels) arise from each portion of this extracapsular arterial ring and penetrate the hip capsule to enter the hip joint. The numerous branches from the lateral ascending cervical artery (which branch from the medial femoral circumflex artery) provide circulation to the greatest portion of the femoral head and neck. After penetrating the hip capsule, the ascending cervical arteries form a second arterial ring that is also usually incomplete. This intra-articular, subsynovial ring is smaller than the extracapsular ring and is located at the border between the articular surface of the femoral head and the femoral neck. These subsynovial vessels are consistently present medially and laterally and less commonly present anteriorly and posteriorly. The epiphyseal branches of these vessels cross the physis on the surface of the femoral head, enter the perichondral ring, and then cross into the epiphysis.
Intraosseous Blood Supply.
The intraosseous blood supply may be compromised by proximal femoral osteotomies or the internal fixation of SCFE. The ascending cervical arteries penetrate the intracapsular femoral neck, with different vessels supplying the metaphysis and epiphysis (160, 161, 163). The intraosseous blood supply of the femoral head is mainly located in its posterior and superior portions, with potential implications for the positioning of hardware (160). The extent of anastomoses between these vessels and the arterial branches of the ligamentum teres (which supply the medial third of the femoral head) appears to be quite limited (160, 161, 163).
NATURAL HISTORY
In the short term, the natural history of the affected hip is one of progressive displacement, followed ultimately by stabilization of the slip and physeal closure. Although all slips must eventually cease progressing, the timing of cessation and the degree of the slip prior to cessation and physeal closure are unpredictable. Most slips progress slowly, although some may have significant, acute progression. The hips with such acute progression are the ones at the highest risk for significant complications.
Bilateral SCFE at the time of initial presentation accounted for approximately 20% of the children with SCFE in recent series (15, 28, 29). It is probable that this frequency will further increase with the increased awareness of the frequency of bilateral involvement and with the ongoing improvements in the imaging of SCFE.
An additional 10% to 20% of patients with SCFE are diagnosed with a contralateral SCFE in adolescence (6, 13, 31, 32, 164). About 80% to 90% of symptomatic, contralateral SCFE cases are diagnosed within 18 months of the diagnosis of the first slip, with 66% to 81% being diagnosed in the first year (15, 28, 125, 165). The average duration between the diagnosis of the first and second slips in metachronous bilateral SCFE has been reported as 1.0 ± 0.8 years (15). Contralateral slips have been reported as late as 4 to 5 years following the initial SCFE (6, 15, 24).
The true frequency of bilateral SCFE at long-term follow-up appears to be approximately 60% (13, 31), although rates of up to 80% have been reported (33). Many of the late contralateral SCFE cases reported in long-term radiographic follow-up are mild, asymptomatic slips (13, 31, 33). These data suggest that if 20% of the patients present with bilateral SCFE, then half of the 80% who present with unilateral SCFE will ultimately have a contralateral SCFE.
In the short term, 61% to 100% of children with endocrinopathies and SCFE have bilateral slips, although metachronous involvement is common (41, 58). Because of this significant short-term risk, prophylactic pinning of the contralateral hip is recommended in patients with SCFE and endocrine disease (41, 58).
In the long term, SCFE puts the hip at significant risk of OA, poorer results being associated with an increasing degree of SCFE (8, 125, 166, 167, 168 and 169). Hagglund et al. (31) reported radiographic
evidence of OA in 27% (28 of 104) of hips with SCFE at long-term follow-up (mean follow-up: 33 years) compared with 9% of control hips (9 of 101). Carney and Weinstein (167) reported a long-term follow-up (mean follow-up: 41 years) of 28 patients with 31 untreated SCFEs (between 1915 and 1952) and correlated the degree of the slips with radiographic and clinical scores. Patients with mild slips fared better than did those with moderate and severe slips with regard to radiographic changes and Iowa hip scores. At long-term follow-up, Iowa hip scores were at least 80 in all 17 hips with mild slips and in 9 of the 14 hips (64%) with moderate or severe slips. There was radiographic evidence of OA in 64% (9 of 14) of the mild slips and in 100% (13 of 13) of the moderate and severe slips.
evidence of OA in 27% (28 of 104) of hips with SCFE at long-term follow-up (mean follow-up: 33 years) compared with 9% of control hips (9 of 101). Carney and Weinstein (167) reported a long-term follow-up (mean follow-up: 41 years) of 28 patients with 31 untreated SCFEs (between 1915 and 1952) and correlated the degree of the slips with radiographic and clinical scores. Patients with mild slips fared better than did those with moderate and severe slips with regard to radiographic changes and Iowa hip scores. At long-term follow-up, Iowa hip scores were at least 80 in all 17 hips with mild slips and in 9 of the 14 hips (64%) with moderate or severe slips. There was radiographic evidence of OA in 64% (9 of 14) of the mild slips and in 100% (13 of 13) of the moderate and severe slips.
Ordeberg et al. (169) reported a 20- to 60-year follow-up (mean follow-up: 37 years) of 49 cases of SCFE who did not undergo primary treatment. They reported that only “a few” patients had restrictions regarding their work or social lives and that only 2 of 49 (4%) had required surgery for arthritis. Limb-length discrepancy (LLD) of at least 2 cm was noted in 31% of the cases. The authors also noted that these results were far superior to a comparable group of patients treated with closed reduction and casting. Jerre (32) noted superior results in untreated patients in Sweden as well.
Previous authors have noted that known cases of SCFE account for 2% to 9% of end-stage hip arthritis (170, 171, 172, 173 and 174). A cadaveric study noted “postslip” morphology in 8% of the skeletons and showed that OA was associated with such morphology (175).
In older case series studies, a significant proportion of adults with “idiopathic” OA have been reported as having a stigma of pediatric hip disease, such as a “pistol grip” deformity. Murray (176) reported an apparent association with SCFE in 40% of the adult hips thought to have degenerative arthritis as evidenced by the so-called tilt deformity of the femoral head. Stulberg et al. (177) reported such deformity in 40% of patients with hip OA and no previously diagnosed hip disease. Stulberg et al. (177), however, noted that the “tilt deformity” did not appear to be unique to SCFE. Resnick (178) has suggested that the “tilt deformity” is not due to SCFE, but is due to the remodeling of the osteoarthritic hip; hence, the underlying etiology of most end-stage hip OA remains unclear.
In summary, 20% of patients with SCFE present with unilateral disease, an additional 10% to 20% develop a contralateral slip during adolescence, and 60% of the patients have bilateral SCFE, which is evident at long-term follow-up. In all the cases of SCFE, OA appears to result, with worse slips being associated with increased rates and severity of the OA. Although SCFE leads to late degenerative changes, most hips function well into their fifth decade or later.
TREATMENT
Once the diagnosis of SCFE is made, the child is admitted to the hospital and is confined to bed until surgery is performed, as has been recommended for decades (23). Under no circumstances should the child be allowed to bear weight once the diagnosis of an acute/unstable SCFE is made, as it may result in ON.
The goals of treatment in SCFE are early detection, prevention of further slipping, and avoidance of complications. Although attention is often focused on the affected hip, care of the unaffected hip (either through careful observation or through prophylactic treatment) cannot be forsaken.
Care of children with SCFE continues to advance along with our understanding of this disease. Increased vigilance and enhanced imaging allow the early detection of SCFE, and percutaneous fixation techniques allow for short hospital stays (or even outpatient surgery). With these enhancements in care, one recent study comparing treatment of children with SCFE at a pediatric hospital to the treatment given at a general hospital reported shorter hospital stays and lower hospital charges at the children’s hospital (179).
As has been noted, SCFE puts the patient at long-term risk of OA, with the risk increasing along with the increase in the degree of slip. In some cases, the outcomes of SCFE (treated or untreated) are so poor that salvage treatment by arthrodesis or arthroplasty may be needed.
Historical Methods
Spica Casting.
The goal of spica casting is to prevent the progression of a SCFE. Although used in the treatment of SCFE for much of the last century, spica casting is now rarely used in the treatment of SCFE. Because most children with SCFE are obese adolescents, use of a spica cast for these children holds little appeal for most patients, their families, and physicians.
Traditionally, spica casting has been associated with high rates of complications (180). Meier et al. (180) reported complications in 14 of 17 hips in which a SCFE (82%) had been treated with spica casting, including 9 cases of chondrolysis (53%), 3 cases of further slip after cast removal (18%), and 2 cases in which a total of 3 pressure sores developed (12%). Chondrolysis has been reported in 14% to 53% of the cases of SCFE treated with spica casting, and it has also been reported in the uninvolved hip following immobilization (32, 180, 181, 182 and 183). ON has commonly been reported with the use of spica casting as well, although most cases of ON appear to be due to the forceful manipulation of the SCFE rather than to the spica cast itself.
Progressive slip occurs in 5% to 18% of cases of SCFE treated with spica casting (180, 182). Although Betz et al. (182) cited only a 3% incidence (1 per 37 hips), the true rate is 5% in their study because they excluded the progression of one additional hip that had been followed up for <2 years.
The duration of casting has often been arbitrary. In the absence of any operative intervention, most proximal femoral physes do not close for a year or more following the diagnosis of SCFE. Most children treated with casting are immobilized for 3 to 4 months (180, 182). Betz et al. (182) noted that spica casts could safely be removed when the
juxtaphyseal metaphyseal radiolucency was no longer visible, and that this occurred by 16 weeks in their patients. Although all patients were immobilized in a cast for periods ranging from 117 to 124 days, Meier reported progressive slips in 18% (3 of 17) of the hips after cast removal (180).
juxtaphyseal metaphyseal radiolucency was no longer visible, and that this occurred by 16 weeks in their patients. Although all patients were immobilized in a cast for periods ranging from 117 to 124 days, Meier reported progressive slips in 18% (3 of 17) of the hips after cast removal (180).
With the advent of current fluoroscopic imaging techniques, cannulated screw systems, and the decrease in operative morbidity, there is little role for nonsurgical treatment in children with SCFE.
Bone Graft Epiphysiodesis.
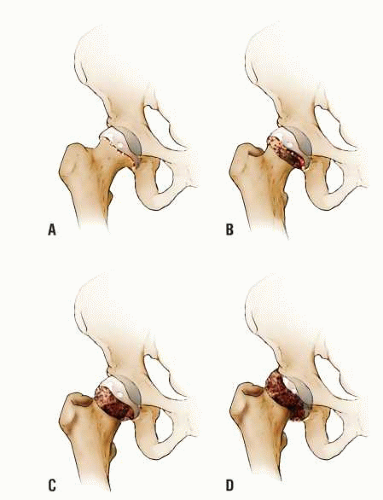
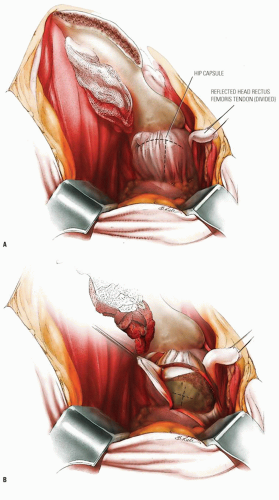
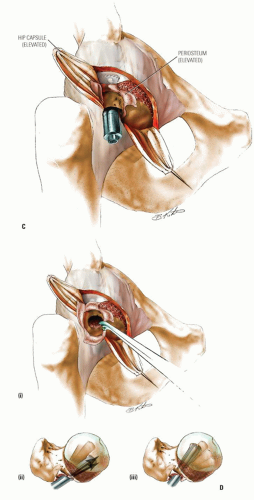
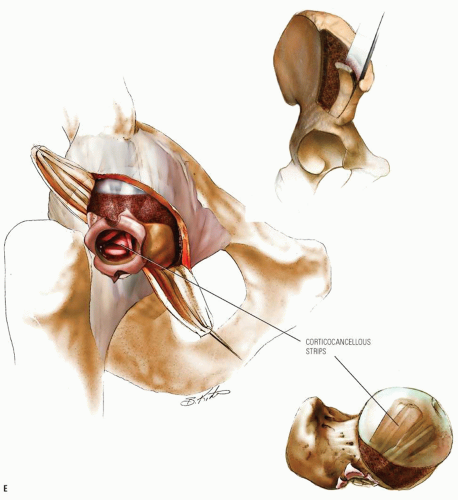
The goal of bone graft epiphysiodesis, as with in situ fixation, is the prevention of slip progression. However, the way in which this is achieved with the two methods differs. Slip progression is prevented with bone graft epiphysiodesis primarily by hastening physeal closure, whereas in situ fixation prevents slip progression primarily by stabilizing the physis. Indications for bone graft epiphysiodesis include acute/unstable or chronic/stable SCFE of any magnitude, although some authors have conceded that cases of mild SCFE are better treated with in situ fixation (184) (Fig. 25-5A-E).
Bone graft epiphysiodesis, which involves drilling across the physis into the epiphysis with placement of bone graft (most commonly autologous bone pegs), was first described in 1931 by Ferguson and Howorth (185). Although reported results have often been good (184, 186, 187, 188, 189, 190, 191, 192, 193 and 194), this operation has been abandoned at many institutions because of potential for morbidity and technical difficulties (195, 196 and 197).
The surgery may be performed through an anterior or an anterolateral approach and may be combined with osteoplasty of the anterior femoral neck (184, 194, 198). A 50-year experience with bone graft epiphysiodesis in 318 cases of SCFE presents this procedure as a “reasonable alternative” for the treatment of SCFE (187). Patients with acute SCFE are placed in a spica cast or brace postoperatively and kept without bearing weight for 6 to 8 weeks. Patients with chronic slips begin touch-down weight bearing 2 to 3 days postoperatively and bear weight progressively as the physeal closure progresses. Some authors have reported the time required until full weight bearing as averaging 10 weeks (193).
As reported in most series, surgical time (excluding casting, when necessary) averages 2 hours (186, 193, 196). Weiner et al. (193) have reported estimated blood loss (EBL) for autologous bone peg epiphysiodesis of at least 200 mL in 52% of patients (25 of 48), and other authors have reported mean EBL ranging from 426 to 800 mL (195, 196, 199). When allograft is used instead of autograft, mean EBL has been reported as 360 mL (186).
Physeal closure following an autograft bone peg epiphysiodesis is reported to occur at 4 to 6 months by most authors (186, 188, 189, 195, 196). In a series of bone peg epiphysiodesis with allograft, a partial physeal closure was noted radiographically after an average of 11 weeks and complete closure after an average of 28 weeks, with physeal closure occurring in the operated hip before it occurred in the unoperated hip in all of the 16 unilateral cases (186).
Complications of this procedure include graft failure, failure to achieve physeal closure, slip progression, heterotopic ossification, lateral femoral cutaneous nerve (LFCN) palsy, donor site morbidity, chondrolysis, and ON. Heterotopic ossification has been reported in up to 69% of patients (196). Despite intraoperative protection of the nerve, Ward and Wood (199) reported LFCN palsy in 10 out of 14 patients (71%) specifically examined for this finding postoperatively. Rao et al. (196) reported transient LFCN palsy in 11% of their patients.
Graft complications following bone peg epiphysiodesis are well described. In two large series with very good results, Adamczyk et al. (187) reported graft resorption with failure of epiphysiodesis in a period of 1 year in 4% of cases (12 of 318 hips), and Howorth reported graft resorption in 2% of cases (4 of 200 hips), with no cases of progressive slip (188). In a series of 17 cases of SCFE, Ward and Wood (199) reported “graft insufficiency,” defined as graft movement, resorption, or fracture, in eight hips (47%). Protrusion of the graft into the hip joint has also been reported (195).
The rate of ON associated with bone peg epiphysiodesis has generally been low, with most reports in the range of 0% to 6%, with higher rates in acute/unstable SCFE (186, 187 and 188, 196, 198, 199). Adamczyk et al. (187) reported an overall rate of 2%, with a risk of 7% in acute slips (3 of 45 cases) and 1.5% in chronic slips (4 of 273 cases). The low rate of ON in bone peg epiphysiodesis is likely due to placement of the grafts from the anterolateral neck and into the center of the epiphysis, thereby avoiding the intraosseous blood supply.
Chondrolysis is reported to occur in 0% to 6% of the cases of SCFE treated with bone peg epiphysiodesis (186, 187 and 188, 196, 199). Most cases of chondrolysis occur in acute/unstable SCFE (187, 196).
Progressive slip has been reported in 0% to 19% of cases following bone peg epiphysiodesis, with the highest risk being in acute SCFE (186, 187 and 188). Although Rao et al. (196) noted a change in the femoral head-shaft angle of at least 5 degrees in 42% of patients (27 of 64), the angle increased in 19% and decreased in 23% of the cases. One presumed reason for slip progression is that the bone graft does not stabilize (and may actually destabilize) the proximal femur as well as does a screw. Another potential cause of progressive slip in these patients is the delayed or incomplete physeal closure.
Femoral neck fracture has also been reported in 0% to 5% of cases following bone peg epiphysiodesis (186, 187, 195). Schmidt et al. (186) reported 2 proximal femoral fractures in a series of 40 bone peg epiphysiodeses (5%), in striking contrast to Adamczyk et al. (187), who reported no fractures in a series of 318 bone peg epiphysiodeses.
Bone graft epiphysiodesis does not have significant advantages relative to in situ fixation of SCFE, although there are significant drawbacks to its use. Children treated with bone graft epiphysiodesis have greater blood loss, increased donor site morbidity, increased risk of nerve palsy, increased risk of slip progression, and are not allowed to bear weight as early as do those children undergoing in situ fixation. Bone graft epiphysiodesis does not appear to have a significant role in the treatment of SCFE at this time.
Technique for Bone Graft Epiphysiodesis for Treatment of SCFE (Fig. 25-5A-E)
Postoperative Care.
A drain can be used at the discretion of the surgeon, but there should be no dead space and little bleeding at the conclusion of the procedure. Those series that reported no further slipping after grafting used a spica cast for immobilization until healing was complete in 8 to 12 weeks. There have been reports of using only crutch protection, not a cast, with an incidence of further slipping in some patients (193).
Current Methods
In Situ Fixation.
The goal of in situ fixation of SCFE is to prevent slip progression. In situ fixation is currently the
preferred initial treatment for most cases of SCFE, both stable and unstable, although the outcome of such treatment differs depending on the slip stability and severity.
preferred initial treatment for most cases of SCFE, both stable and unstable, although the outcome of such treatment differs depending on the slip stability and severity.
Over a period of more than 50 years of in situ fixation for SCFE, surgical techniques, implants, and imaging techniques have evolved significantly (200). Early fixation was with large nail-type devices, followed by pin fixation, which have since been replaced by cannulated screw systems in most centers. Because of the wide availability of fluoroscopic imaging, the ability to optimally position the fixation devices has improved as well. Cannulated screw systems now allow these procedures to be performed percutaneously.
The surgery may be performed on either a fracture table or a radiolucent table (200, 201). Use of a fracture table allows a true lateral radiograph to be obtained, although the quality of such images in obese patients is often suboptimal and this setup requires the presence of a technician to rotate the fluoroscope. In contrast, with the patient on a radiolucent table, a technician is not needed, as the fluoroscope may be left in one position and it is easy to obtain a higher quality frog lateral radiograph; however, a true lateral can only be obtained by moving the patient. In addition, the guide wire for percutaneous fixation may be bent as the hip is rotated. For an unstable SCFE, the radiolucent table may be preferable in order to limit traction that may forcefully reduce the femoral head.
Understanding the three-dimensional pathoanatomy of the SCFE is essential for understanding how to position the hardware optimally and minimize complications. As noted previously, the proximal femoral neck and shaft migrate anteriorly and rotate externally in most SCFE. As a result, a greater portion of the femoral head is located posterior to the femoral neck as the SCFE progresses. In very severe cases of SCFE, the entire femoral head is posterior to the femoral neck.
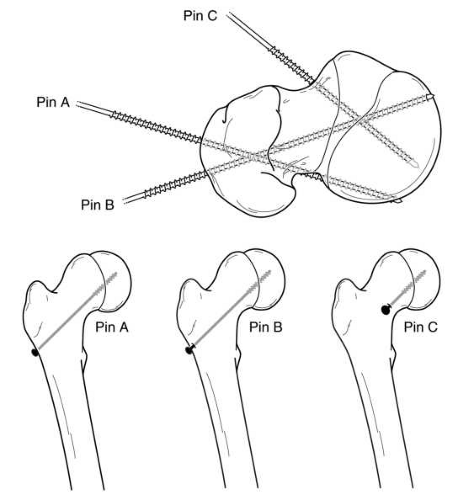
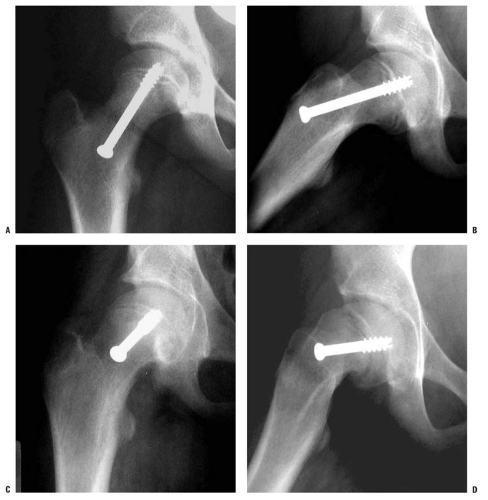
When placing the in situ pin, the goals are to stabilize the physis with minimal hardware, avoid ON by avoiding pin penetration of the posterior femoral neck and pin placement in the anterior-superior head, avoid chondrolysis by avoiding pin penetration into the joint, and finally avoid screw impingement by avoiding intra-articular placement of screw entry point. Because of the direction of the slip, fixation should be inserted from the anterior femoral neck in most cases in order to allow fixation perpendicular to the physis and to prevent hardware penetration through the posterior femoral neck (144, 202) (Fig. 25-6). However, in very severe cases, the hardware may need to be inserted in a directly anterior-to-posterior direction (Fig. 25-7), which may cause screw head impingement and articular damage. When possible, the screw insertion point should be lateral to the intertrochanteric line even though the screw may not be perpendicular to the physis (203). However, insertion of hardware from the far lateral cortex (as is done in the pinning of adult hip fractures) will generally result in one or more of the following problems: poor biomechanical alignment of the hardware (very oblique rather than perpendicular to the physis), purchase of the hardware in only a small portion of the femoral head, joint penetration, hardware exiting the posterior femoral neck before entering the femoral head, and creation of stress risers on the tension side of the proximal femur. Common sequelae with a lateral starting point are that the hardware either entirely misses or engages only a small portion of the anterior femoral head, and that such hardware also often penetrates the joint surface. If the hardware exits the posterior femoral neck before entering the femoral head, as has been reported in up to 6% of cases (103, 204), the extraosseous blood supply to the femoral head are at risk, thereby increasing the risk of ON.
Ideally, the fixation device should be located in the center of the proximal femoral epiphysis on both the anteroposterior and lateral views and should be perpendicular to the physis in both views as well (205, 206). This so-called center-center position minimizes pin penetration into the joint and provides optimal fixation of the physis (68, 207, 208 and 209). Additionally, it minimizes superior or posterior placement of the screw, which may place at risk the intraosseous blood supply within the head. One of the significant difficulties in pinning SCFE is the three-dimensional interpretation of intraoperative radiographic images. Walters and Simon (207) alerted the orthopaedic community to the risk of unrecognized pin penetration in cases of SCFE treated with in situ fixation, and the associated risk of chondrolysis. They
demonstrated that a “blind spot” can exist radiographically, since a protruding pin may appear to be located within the femoral head on both anteroposterior and lateral views (207). Other authors have described a geometric analysis of the blind spot, although this technique is rarely used (210).
demonstrated that a “blind spot” can exist radiographically, since a protruding pin may appear to be located within the femoral head on both anteroposterior and lateral views (207). Other authors have described a geometric analysis of the blind spot, although this technique is rarely used (210).
In practice, the operative hip is taken through a full range of motion while using fluoroscopy. This can be done throughout the procedure if a radiolucent table is used. If a fracture table is used, this can only be done following removal of traction on the operated leg. The “approach-withdraw phenomenon” described by Moseley (211) is when the fluoroscopic appearance of the implanted hardware approaches the subchondral bone and then moves away from it. When the hardware reaches the apex of this arc and then begins to recede,
the point of maximal proximity to the subchondral bone has been reached, and this distance should be measured. Center-center pins are left 5 to 6 mm from the subchondral bone (corrected for magnification), while other pins are left 10 mm from the subchondral bone (207). Poor hardware position has been noted to correlate with poor clinical outcomes (202, 212).
the point of maximal proximity to the subchondral bone has been reached, and this distance should be measured. Center-center pins are left 5 to 6 mm from the subchondral bone (corrected for magnification), while other pins are left 10 mm from the subchondral bone (207). Poor hardware position has been noted to correlate with poor clinical outcomes (202, 212).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree