Skin Lesions
D. Scot Malay
Marija Ugrinich
SITES OF ORIGIN
The skin consists of the epidermis, the dermis, and the subcutaneous layer (hypodermis or subcutis) and is the largest organ of the body. The skin contains sensory and temperature receptors, and it serves as a protective envelope that resists mechanical, infectious, radiation, and thermal trauma. It also functions in thermoregulation and in the production of vitamin D and cytokines. Skin appendages include eccrine glands, which are most abundant on the palms and soles and axillae, hair follicles, and sebaceous glands, which are found on all skin surfaces except the palms and soles and empty into the hair follicle. Any cell contained within the various skin components can become neoplastic and can result in the formation of a tumor. Malignant tumors of epidermal germ cell origin are termed carcinomas, whereas those of mesenchymal origin are referred to as sarcomas.
ETIOLOGY AND PATHOGENESIS
Ionizing radiation and recurrent cutaneous irritation, whether chemical, mechanical, or thermal, are thought to be potential causes of skin cancer. Exposure to ultraviolet radiation and skin type are two factors influencing the development of skin cancer (1). Generally, less pigmented skin is more sensitive to ultraviolet radiation, and a light-skinned person is more likely to develop skin cancer than a more darkly pigmented person. Basal cell carcinoma and malignant melanoma are generally understood to be the most common forms of skin cancer that affect lightly pigmented skin. Chemical carcinogens, such as organic tars, and viruses, specifically human papillomavirus, have been suspected as causes of cutaneous malignancy. Metastasis to the skin by any tumor originating in tissues other than the skin itself is rare and is a poor prognostic indicator. Cutaneous malignancies, such as malignant melanoma and squamous cell carcinoma, often metastasize to distant areas of the skin. Etiologic considerations for the development of soft tissue sarcomas, as compared with skin tumors of epithelial origin, include genetic predisposition, chemical carcinogenesis, posttraumatic malignant transformation, and ionizing radiation.
DIAGNOSIS AND MANAGEMENT
Any enlargement of tissue, whether edematous, hypertrophic, or neoplastic, can be referred to, in a general sense, as a tumor. Even experienced clinicians, as well as seasoned pathologists, can have difficulty in identifying the exact cellular origin and biology of a skin tumor, and accurate diagnosis usually hinges on microscopic pathologic inspection. The clinical evaluation of these lesions includes a history and physical examination that entails a thorough skin examination, as well as inspection of the regional lymph nodes and the cutaneous surfaces between the lesion in question and the regional lymph node basin. The lymphatic vessels that function over the majority of the foot drain into the inguinal lymph nodes, and the lymphatic vessels from only a small, distal area of the dorsolateral aspect of the foot drain into the popliteal nodes.
General considerations in the assessment of a skin neoplasm include the following: the lesion’s color, size, shape, or elevation or recent changes in any of the foregoing features; the lesion’s location, superficial (freely movable below or within the skin) or deep (fixed) to the deep fascia (muscle fascia); the lesion’s vascularity or pulsatile nature; and the status of the popliteal and inguinal lymph nodes (tender or enlarged). Moreover, the presence of symptoms such as pain, pruritus, hemorrhage, sensory or motor disturbance, constitutional malaise, or fever or weight loss should be taken into consideration. When evaluating a neoplasm, a high index of suspicion should be maintained for potential malignancy. Any lesion, even one thought to be a persistent pyogenic granuloma, chronic onychocryptosis, a resistant verruca, or simply a benign subcutaneous mass, that does not respond to reasonable therapy in a timely fashion may require more thorough evaluation. Closer inspection may involve radiography, magnetic resonance imaging (MRI), computed tomography (CT), ultrasonography, positron emission tomography (PET), laboratory testing, or biopsy. Clinical laboratory testing, including a complete blood count and differential cell analysis, biochemical profile, suspected tumor antigen testing, and erythrocyte sedimentation rate, may be helpful. Keep in mind that procuring a suitable tissue specimen in the clinic may be the easiest, most rapid, and most diagnostic method, in comparison to any of the other diagnostic modalities mentioned above. Furthermore, as a general rule, any lesion identified as malignant warrants oncologic consultation and systemic evaluation for lymph node, lung, gastrointestinal, bone, or other sites of potential regional dissemination or metastasis. After making the diagnosis, then appropriate treatment may range from simple observation to radical excision, and plastic surgical wound coverage techniques may be required to subsequently achieve wound closure.
BIOPSY TECHNIQUES
Biopsy is undertaken in an effort to confirm the cells of origin and thereby accurately identify the tumor. Various biopsy techniques are available including needle aspiration and shave, punch, incisional, and excisional biopsy. Needle biopsy, including fine-needle aspiration of superficial lesions, can be helpful if multiple core specimens are obtained from different sites within the lesion. Incisional biopsy through the midportion of the soft tissue mass is the preferred method of biopsy for most skin tumors of the lower extremity. During an incisional biopsy for suspected malignancy, the biopsy channel to the lesion should be oriented longitudinally in line with the suspicious
mass and within a region that is to be fully excised with subsequent definitive surgical excision. The surgeon should also avoid dissection into adjacent fascial compartments, in an effort to leave these structures intact so they can persist as natural anatomic barriers to the spread of malignant cells. The surgeon can, in many cases of carcinoma or sarcoma, preserve adjacent muscle compartments protected by intact deep fascia only when the natural anatomic barriers have been preserved. Attention to such detail may be the difference between muscle compartment resection from the foot into the leg and belowknee or above-knee amputation or metastasis of the cancer.
mass and within a region that is to be fully excised with subsequent definitive surgical excision. The surgeon should also avoid dissection into adjacent fascial compartments, in an effort to leave these structures intact so they can persist as natural anatomic barriers to the spread of malignant cells. The surgeon can, in many cases of carcinoma or sarcoma, preserve adjacent muscle compartments protected by intact deep fascia only when the natural anatomic barriers have been preserved. Attention to such detail may be the difference between muscle compartment resection from the foot into the leg and belowknee or above-knee amputation or metastasis of the cancer.
An incisional biopsy removes only a selected portion of the lesion in question, whereas an excisional biopsy theoretically removes the entire lesion. Obviously, a margin of supposedly “normal” surrounding tissue is excised along with the lesion in question when an excisional biopsy is performed. Actual pathologic assessment of the margins of the biopsy is necessary to determine whether a “clean” margin was created. When performing an incisional biopsy, the surgeon should select representative portions of the lesion, and more than one sample may be procured. It is important to identify the samples accurately relative to position within the lesion, and a diagram may be helpful in this regard. The pathologist would also benefit from review of the diagram when examining a difficult lesion. The surgeon should not struggle to procure a margin of normalappearing tissue in the incisional specimen at the risk of missing a representative lesion. Scabs, crusts, and vesicles should be preserved in the specimen and not destroyed in the surgical preparation of the skin. Most skin biopsies can be performed with the use of local anesthesia, and dilute epinephrine (1:100,000 or 1:200,000 dilution) can be used to aid hemostasis if it is not contraindicated. The local anesthetic agent should not be infiltrated directly into the lesion for fear of spreading potential malignancy or infection, even though it has been shown that direct injection below or through a lesion appears to cause no significant microscopic alteration. After establishment of local anesthesia, the area in question is then prepared and draped in the usual sterile fashion before biopsy.
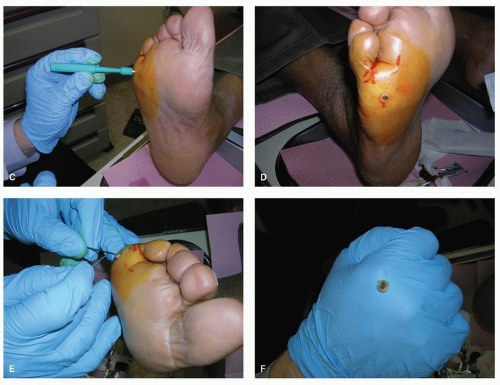
The punch biopsy is a convenient and effective method of tissue extraction and may be either incisional (usually) or excisional when dealing with a large punch and a smaller lesion (Fig. 91.1). In the lower extremity, the 4-mm punch is typically employed, and standard punch sets include punches ranging from 2 to 8 mm. Removal of a specimen less than 4 mm in diameter may allow the histologic confirmation of a tumor; however, it is generally considered inadequate for the accurate diagnosis of an inflammatory process. When the punch is used, the skin surrounding the lesions should be stretched taut perpendicular to the wrinkle lines before the circular punch is inserted. The punch biopsy device is firmly pressed into the lesion with a rotary back-and-forth cutting motion until it is well into the subcutaneous tissue, thereby providing a full-thickness skin biopsy. To remove the cylindrical specimen from the skin, the biopsy plug is gently grasped with forceps, or a 27-gauge needle, the base is cut with scissors or scalpel, and the sample is placed into 10% formalin. Simple pressure is adequate for hemostasis. A punch biopsy defect 4 mm in diameter or greater is usually sutured, whereas a defect smaller than 4 mm heals by secondary intention with adhesive skin strips and a sterile dressing. A linear defect usually heals more readily than a round defect.
The shave biopsy is used to remove that portion of the skin elevated above the plane of surrounding tissue and is useful for biopsy or removal of many benign epidermal growths. As a general rule, a shave biopsy will not provide a definitive diagnosis in regard to the depth of a particular skin lesion; however, it can be useful in identifying the presence of malignancy, or other histopathologic abnormalities, in the more superficial layers of the skin. Therefore, a shave biopsy will often inform of the presence or absence of pathology; however, it usually will not enable the histopathologic microstaging of a particular lesion, and a more definitive biopsy will be needed to determine the microstage of the disease. The primary value of the shave biopsy lies in its ease of use and the ability to ascertain the presence or absence of abnormal, perhaps malignant, cells in the superficial portion of the skin. In fact, it can be particularly useful as
a convenient procedure for diagnosis of basal cell epithelioma (basal cell carcinoma). The preparation of the skin is as noted above for incisional biopsy, and a no. 15 blade is used to shave the lesions and a margin of apparently normal surrounding tissue, and pressure is applied to effect hemostasis. The specimen is labeled and retained in 10% formalin.
a convenient procedure for diagnosis of basal cell epithelioma (basal cell carcinoma). The preparation of the skin is as noted above for incisional biopsy, and a no. 15 blade is used to shave the lesions and a margin of apparently normal surrounding tissue, and pressure is applied to effect hemostasis. The specimen is labeled and retained in 10% formalin.
Curettage is a useful technique, distinguishable from the shave biopsy, for removal of cutaneous lesions such as warts, seborrheic keratosis (SK), and even malignant lesions such as basal cell carcinoma or squamous cell carcinoma. The curette is a spoon-like instrument with a sharp rim. Typically, the 4-mm curette is used. A variation on this technique, termed excochleation, using a blunt instrument such as a Freer elevator, is useful for removal of a lesion that has a clear and separable margin with the adjacent normal tissue. A verruca is ideal for excochleation, and this method provides an excellent specimen for biopsy and prepares the base for ablative surgery (acid, cryogen, or laser) if desired.
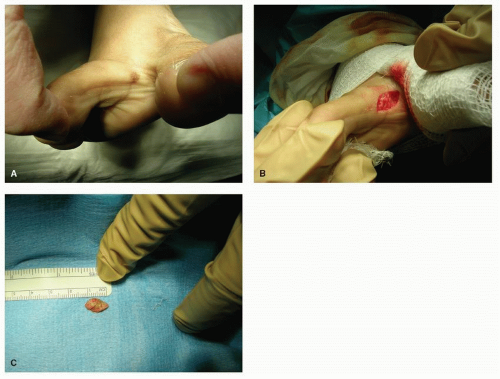
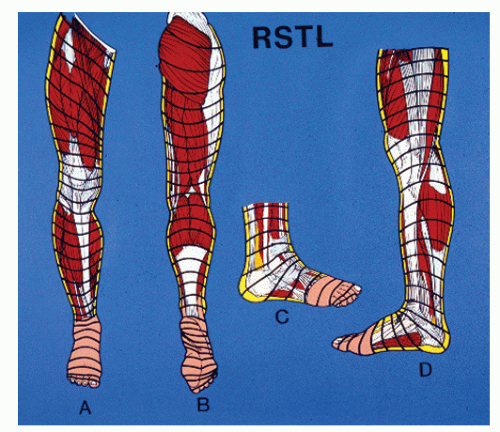
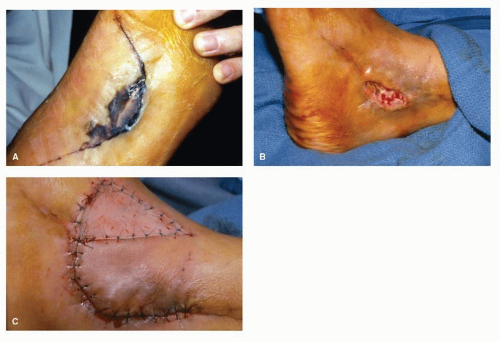
Surgical excisional biopsy should be considered whenever the lesion displays active (spreading) margins and the border with normal-appearing skin is to be surveyed, when the lesion is friable or sclerotic, or whenever it is necessary to include full-thickness skin to the level of, and including a portion of, the subcutaneous fat layer (e.g., in suspected panniculitis, erythema nodosum, or melanoma) (Fig. 91.2). In theory, excisional biopsy can be curative, and it may be used whenever excision of a lesion is desired. Local anesthesia and preparation of the skin are achieved as described earlier for incisional biopsy. The most common technique involves the use of two semielliptical incisions to create a wedge of intervening tissue to be excised. The length of the ellipse should be three to four times the width, with the long axis of the wedge parallel to the relaxed skin tension lines (RSTLs) when feasible and practical. Surrounding margins may be undermined to decrease tension with closure, and weight-bearing on the plantar skin is usually avoided for 2 to 4 weeks, depending on the size of the wound and progress of healing (Fig. 91.3).
When malignancy is suspected, it is important to avoid contamination of surrounding tissue planes with cellular elements of the lesion in question. This is such an important surgical consideration that it is reiterated at this time. A malignant lesion can often be adequately eradicated with wide excision,
compartment resection, or amputation, and violation of adjacent soft tissue barriers at the time of initial biopsy may make it necessary for subsequent definitive therapy to be more ablative than it would otherwise have been. A frozen section may be used to identify the presence or absence of tumor in a particular specimen and perhaps to yield identification of the specific cell type responsible for the tumor. It is often difficult to identify the specific tumor pathologically solely from a frozen section specimen; however, the presence of undifferentiated and metaplastic cells can usually be determined. A frozen section must be planned in advance with the pathologist and operating room personnel, and the surgeon should be prepared to discuss the operative specimen with the pathologist preparing and interpreting the prepared specimen. If a local excisional biopsy is performed and the frozen section pathology report yields a diagnosis of malignancy, then adequately wide excision should be attempted only for extremely small and well-defined lesions. By definition, sarcomas are invasive and are not well differentiated from adjacent soft tissues. If malignancy is identified or strongly suggested with the frozen section, then the surgeon should discuss the diagnosis with the pathologist and determine whether or not additional biopsy specimens should be procured. Thereafter, the best course may entail simply closing the wound and proceeding with oncologic consultation and preparation for definitive and adjunct medical and surgical interventions. Once again, cross-contamination of uninvolved tissues by means of over aggressive or unnecessarily extensive dissection for the biopsy can be detrimental to the long-term health of the patient, and this is a particularly prevalent complication associated with well-intentioned efforts to fully excise a lesion that actually turns out to be a sarcoma. Carcinomas localized to the skin may be more readily isolated and amenable to definitive excision and eradication at the time of excisional biopsy. Regardless of the ultimate diagnosis, patients that undergo frozen section biopsy should be prepared to have to return to the operating room for a more definitive biopsy at a later date.
compartment resection, or amputation, and violation of adjacent soft tissue barriers at the time of initial biopsy may make it necessary for subsequent definitive therapy to be more ablative than it would otherwise have been. A frozen section may be used to identify the presence or absence of tumor in a particular specimen and perhaps to yield identification of the specific cell type responsible for the tumor. It is often difficult to identify the specific tumor pathologically solely from a frozen section specimen; however, the presence of undifferentiated and metaplastic cells can usually be determined. A frozen section must be planned in advance with the pathologist and operating room personnel, and the surgeon should be prepared to discuss the operative specimen with the pathologist preparing and interpreting the prepared specimen. If a local excisional biopsy is performed and the frozen section pathology report yields a diagnosis of malignancy, then adequately wide excision should be attempted only for extremely small and well-defined lesions. By definition, sarcomas are invasive and are not well differentiated from adjacent soft tissues. If malignancy is identified or strongly suggested with the frozen section, then the surgeon should discuss the diagnosis with the pathologist and determine whether or not additional biopsy specimens should be procured. Thereafter, the best course may entail simply closing the wound and proceeding with oncologic consultation and preparation for definitive and adjunct medical and surgical interventions. Once again, cross-contamination of uninvolved tissues by means of over aggressive or unnecessarily extensive dissection for the biopsy can be detrimental to the long-term health of the patient, and this is a particularly prevalent complication associated with well-intentioned efforts to fully excise a lesion that actually turns out to be a sarcoma. Carcinomas localized to the skin may be more readily isolated and amenable to definitive excision and eradication at the time of excisional biopsy. Regardless of the ultimate diagnosis, patients that undergo frozen section biopsy should be prepared to have to return to the operating room for a more definitive biopsy at a later date.
Ultimately, definitive treatment is determined based on clinical (local, regional lymph nodes, metastasis) and microscopic staging. Difficult histologic diagnoses may require further tissue analyses, such as histochemical staining, ultrastructural inspection, antibodies to keratin or the presence of epithelial membrane antigen or carcinoembryonic antigen, or, if melanoma is suspected, antibodies to S-100 proteins and NKIC3. An appropriate treatment regimen, such as wide excision and coverage with a muscle flap, or amputation, is subsequently determined by oncologic consultation and additional testing to ascertain the clinical stage of the tumor. Additional radiographs, CT and/or MRI and/or PET scans of the extremity and the lungs, and perhaps of the liver and spleen, along with other tumor-specific tests, may also be indicated. Adjuvant radiation or chemotherapy may be administered before and after definitive excision, and the oncologist determines the use of such
therapies. After biopsy and oncologic workup, definitive surgery for eradication of the lesion is planned and carried out in a timely fashion.
therapies. After biopsy and oncologic workup, definitive surgery for eradication of the lesion is planned and carried out in a timely fashion.
Various treatment options are available for destruction of skin lesions. Curettage and electrodessication are commonly used, and one starts with debulking the lesion to apparently normal skin or subcutaneous tissue, followed by destruction of any remaining microscopic tumor with high-frequency (500,000 to 1,000,000 Hz) electrodessication radiosurgery. Electromagnetic energy is delivered to the tissues by means of a highly damped alternating current across a potential difference of greater than 2,000 V by a low current of 100 to 1,000 mA. Low current enables accurate application of the radiofrequency energy, and lateral heat dissipation through surrounding tissues denatures proteins and dehydrates cells. The goal is to achieve a 2- to 3-mm ring of destruction about the lesion.
Cryotherapy with liquid nitrogen is another treatment option. Liquid nitrogen, which is -195°C, is useful for lesions up to 2 cm in diameter and, once again, a 2- to 3-mm ring of destruction about the lesion is usually preferred. Moreover, cryotherapy is most effective when two freeze-thaw cycles are employed. Cryotherapy, as compared with electrodessication, is preferred in patients with implanted cardiac pacemakers and those with hypersensitivity to local anesthetic agents.
Chemotherapy, immunotherapy, and radiation therapy may also be useful in the primary treatment of skin tumors. Various agents, including podophyllin, salicylic acid, cantharidin, 5-fluorouracil, and retinoids, have been employed. These agents, however, are used primarily as adjuncts to more definitive and effective destructive measures.
Definitive surgical excision with an appropriate tissue margin is generally considered the most destructive form of tumor
ablation. A wide margin, or even amputation, is usually indicated for malignant lesions of the lower extremity. Excisional biopsy has been described earlier. Plastic surgical consultation and comanagement may be needed to cover a defect created by wide excision effectively, and a free muscle flap in conjunction with skin grafting may be indicated. For facial lesions, and those in which minimal destruction of surrounding tissues is important for functional as well as cosmetic reasons, the Mohs micrographic surgical technique may be applicable (2,3). This technique enables the surgeon to excise the cancer under microscopic control, with each successive layer of excised tissue inspected for undifferentiated cells. Absence of microscopic evidence of cancer cells indicates that a satisfactory depth and width of excision have been achieved.
ablation. A wide margin, or even amputation, is usually indicated for malignant lesions of the lower extremity. Excisional biopsy has been described earlier. Plastic surgical consultation and comanagement may be needed to cover a defect created by wide excision effectively, and a free muscle flap in conjunction with skin grafting may be indicated. For facial lesions, and those in which minimal destruction of surrounding tissues is important for functional as well as cosmetic reasons, the Mohs micrographic surgical technique may be applicable (2,3). This technique enables the surgeon to excise the cancer under microscopic control, with each successive layer of excised tissue inspected for undifferentiated cells. Absence of microscopic evidence of cancer cells indicates that a satisfactory depth and width of excision have been achieved.
Regional lymph node dissection, performed by the oncologic surgeon, is necessary when sentinel node biopsy, or dissection without dye assistance, indicates nodal involvement with cancer cells. Adjunct regional limb perfusion with chemotherapeutic agent, or systemic chemotherapy or radiation of the part, may be indicated and undertaken based on oncologic management recommendations. Similarly, ongoing surveillance and medical management are maintained based on oncologic diagnosis and guidelines.
PLASTIC SURGERY TECHNIQUES USED IN THE TREATMENT OF SKIN LESIONS
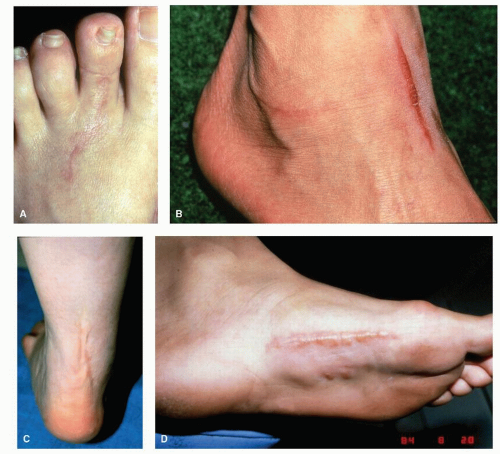
Elective skin incision planning and execution should take into consideration the planned biopsy and exposure of underlying target tissues, preservation of vital structures, and RSTLs (Fig. 91.4). As long as an appropriate biopsy specimen is obtained and adequate exposure is not compromised, the incision should be made parallel to RSTLs. When a transverse incision fails to yield adequate exposure, an incision that is oblique to RSTLs is considered better than one perpendicular to RSTLs, with respect to gapping and scar formation. Difficulty may arise when RSTLs are in a transverse direction and the desired exposure needs to be longitudinal, as in the case of excision of the plantar fascia for treatment of fibromatosis. In such a case, a zigzag incision can be used to effect longitudinal exposure while remaining oblique to RSTLs over the short segments of the zigzag pattern.
Skin wounds that are surgically (or traumatically) created can be closed in a variety of ways. Primary intention closure involves direct wound margin reapproximation secured with skin sutures, effects immediate closure that seals with epithelium after 24 to 48 hours, and usually results in a fine-line scar. Primary skin closure requires wound margin mobility that allows approximation of the edges. Secondary intention closure involves wound granulation, contraction, and epithelialization and slowly progresses over several days to weeks, depending upon local and systemic factors. This is often the preferred method of closure following incision and drainage of an abscess or osteomyelitis, or when the wound displays chronic contamination and necrosis, such as an ulcer in a debilitated patient. A small open wound, created by flap transposition, may be allowed to heal by secondary intention. Tertiary intention (delayed primary) closure involves an initial period of secondary intention granulation healing, followed by additional surgical wound débridement and primary intention suture closure. Primary, secondary, or tertiary intention healing is the preferred methods of wound closure and should be attempted whenever the clinical situation indicates their utility. Generally, other methods of closure, such as skin plasties, grafts, and flaps, entail a greater risk of dehiscence, dysvascularity, or other complication and should be reserved for those instances when more simple methods are deemed inadequate.
Skin grafts consist of epidermis and varying thicknesses of dermis. The graft is said to “take” when it has successfully revascularized and effectively covered the recipient site. The thicker the graft, the more difficult it is to achieve “take.” Contrarily, a thinner graft usually takes more rapidly. Moreover, thicker grafts are more durable and contract less during the healing phase, while thinner grafts are less durable and contract considerably more. Skin grafts are most frequently autogenous, wherein the patient serves as his/her own donor. For podiatric reconstruction, the thigh and buttock, or the anterior aspect of the abdomen, can serve as donor skin graft procurement sites. Skin grafts can also be allogeneic, or from another individual of the same species; xenogeneic, in which the donor tissue originates in a different species (commonly porcine); or isogeneic, wherein the donor is an identical twin. By definition, a graft is detached completely when transferred from the donor site to the recipient bed. Skin graft healing occurs in three phases: (a) plasmatic phase—this involves fibrin adhesion between the graft and recipient surface and occurs during the first 24 to 48 hours; (b) inosculation phase—microvasculature traverses fibrin layer, between 48 and 72 hours or longer; and (c) reorganization/reinnervation—collagenation, remodeling, and cutaneous nerve ingrowth—which occurs weeks to months after graft placement.
A split-thickness skin graft (STSG) can be thin, intermediate thickness, or thick. A thin STSG measures 0.008 to 0.012 inches in thickness, while an intermediate STSG measures 0.012 to 0.016 inches in thickness, and a thick STSG measures 0.016 to 0.020 inches in thickness. Thin STSGs contract as much as 50% to 70% and are often used as temporary coverage of large wounds. Intermediate STSGs are most versatile and frequently used in coverage of foot and ankle wounds. Thick STSGs only
contract about 10% to 15%; however, they demand markedly increased recipient bed vascularity and have a higher risk of failure. Intermediate and thick STSGs can be used to cover weight-bearing and contact areas.
contract about 10% to 15%; however, they demand markedly increased recipient bed vascularity and have a higher risk of failure. Intermediate and thick STSGs can be used to cover weight-bearing and contact areas.
A full-thickness skin graft (FTSG) contains the entire epidermis and dermis, including dermal appendages such as sweat glands and hair follicles. The subcutaneous fat and superficial fascia are not included in an FTSG. FTSGs are very durable and contract minimally; however, they take rather poorly in comparison to STSGs. An FTSG should be considered for coverage of a weight-bearing surface and can be harvested from a pinch of skin over the sinus tarsi, anterior ankle, or medial arch. The inguinal region and popliteal fossa are also potential donor sites.
The recipient bed must be prepared to increase the rate of take, and this should be done prior to harvesting the graft. The recipient site must be free of fibrosis, necrosis, infection, and active hemorrhage. A beefy red, confluent granulation tissue base is the ideal recipient surface. Bare tendon, bone, and cartilage are inadequately vascularized for graft support, and a period of secondary intention healing is required to allow for the formation of a granulation tissue surface. The graft is harvested (procured) from the donor site manually using a Goulain or Humby knife or via use of a pneumatic-powered Brown or Padgett dermatome. Although the graft can be stored for up to 21 days in lactated Ringer’s solution at 0°C to 5°C after procurement, attention should focus on getting the graft to the recipient site in a rapid (immediate) and efficient fashion. The graft may be incised in a fashion similar to pie crusting, wherein small incisions are made to allow expansion and seroma drainage. For large recipient sites, the graft may also be meshed in a 1:1.5 ratio using the graft mesher to increase the area of coverage and enhance drainage of wound transudate through the mesh incisions. Mesh ratios of 1:3, 1:6, and 1:9 can also be achieved; however, these ratios make for sparse coverage of the recipient site and are only used when a large surface requires coverage. Once harvested, the graft is placed in contact with the recipient bed, maximizing contact and eliminating dead space. The graft is then secured at its margins with several simple interrupted stitches of 4-0 or 5-0 multifilament absorbable suture materials. A small amount of excess graft may overlap adjacent intact skin margins. The graft-recipient interface is then secured with a tie-over stent dressing. The tie-over stent dressing employs the use of several evenly spaced nylon sutures, alternating with simple interrupted stitches securing the graft at its perimeter, which consists of the entire length of suture material. A fine mesh nonadherent gauze is then placed over the graft, and fluffed gauze or mineral oil-impregnated cotton balls are then placed upon the nonadherent surface, after which the free margins of the nonadherent gauze are folded over the fluff and the long suture ends are tied over the bandage to secure it with gentle pressure over the graft. The patient is kept at bed rest, and the dressing is left in place for at least 3 days before redressing and wound inspection. The graft donor site is dressed with nonadherent gauze, after assuring hemostasis (topical thrombin spray can be used), and redressed after 48 to 72 hours or longer, depending upon the surgeon’s preference. Skin graft complications develop secondary to disruption of the graft-recipient interface, with resultant failure to achieve revascularization. Causes include seroma formation, infection, and inadequate immobilization. A dysvascular recipient site is doomed to failure.
Skin flaps (Fig. 91.5) differ from grafts in that a vascular pedicle is maintained or, via microsurgical reanastomosis, reconstructed. Flaps are defined as either local or distant. Local flaps are mobilized from adjacent skin and are of either the rotational or advancement type. Rotational flaps are semicircular and are mobilized about a pivot point where the flap is attached to its pedicle. A larger semicircle imparts less tension on the pivot, and tension can also be alleviated at the pivot by means of a back cut or creation of a Burow triangle. The single and bilobed rotation flaps are commonly used for coverage of small pedal skin defects. Advancement flaps are mobilized via direct extension without rotation and include the Y-V, V-Y, single, and bipedicle flaps. Distant flaps originate from a vascular pedicle in one area of the body and, via maintenance of the pedicle or sectioning with subsequent microvascular reanastomosis, are used to cover a defect elsewhere. For example, a crossed leg flap may use skin mobilized from the contralateral calf to cover a pedal defect, or a section of latissimus dorsi, complete with its arterial supply and overlying fascia and skin, may be harvested to cover a pedal defect. In the crossed leg technique, the calf donor skin remains attached at its pedicle while the legs are skeletally fixated and the flap sutured to the contralateral pedal recipient site. Once the flap has healed and attached to the recipient bed, the donor side pedicle is sectioned and the skeletal fixation removed. Nowadays, it is more common to use a microvascular free flap rather than the crossed leg method. The serratus free flap is commonly used by plastic reconstructive surgeons to cover large pedal defects and requires microvascular reanastomosis and coverage of the muscle with an STSG. In the foot and ankle, defects larger than 2.5 cm2 are generally covered with a skin graft, while smaller defects are amenable to use of a local skin flap. Flaps are advantageous for full-thickness defects, poorly vascularized recipient wounds, and coverage of bony prominences or contact areas. Sensation can also be restored when an innervated flap is used.
Skin flaps are further classified according to their blood supply. Random-pattern skin flaps are perfused by the random dermal-subdermal plexus of vessels and require a pedicle width at least equal to the length of the flap. The Z-plasty and V-Y plasty are techniques that create random-pattern, local, rotational, or advancement skin flaps. Axial pattern skin flaps are supplied by an identifiable (Doppler) cutaneous artery, such as the lateral calcaneal artery flap used to cover heel defects, and can be rather long in comparison to the pedicle width. Axial pattern flaps can be of the island design or actually distant flaps when the vascular origin is sectioned and later reanastomosed at the recipient site.
Skin plasties employ local flaps and are used to rearrange the direction and tension, as well as the volume, of skin. Scar tissue and contracture can be redirected and lengthened with the Z-plasty. The arms of the Z are of equal length, with the apices forming an angle of 60 degrees, to achieve approximately 75% increase in skin length with resultant decreased tension. Multiple Z-plasties, effecting a W-plasty, can be combined to further elongate skin and relieve tension. The V-Y (V-to-Y) plasty results in skin lengthening after flap mobilization. The skin defined by the V incision is mobilized and elongated, and the resultant wound is sutured closed in the shape of a Y. As the base of the V is made wider, the vascular pedicle is increased. Skin can also be shortened or reduced by means of a Y-V plasty. Redundant skin can be excised via the creation of an elliptical wedge using two semielliptical skin incisions. This is often
useful in digital surgery, in particular when derotation of a frontal plane deformity is desired.
useful in digital surgery, in particular when derotation of a frontal plane deformity is desired.
Fasciocutaneous flaps can be useful in the leg, in particular for coverage of defects about the Achilles tendon. Muscle flaps are useful for coverage of weight-bearing or contact areas of the foot and ankle, and they convey excellent vascularity and provide a robust base over which skin can grow. Identification and preservation of the muscle’s vascular supply is critical to the success of the flap. The heel and first metatarsal head area are amenable to coverage with flexor digitorum brevis and/or flexor hallucis brevis, and the medial and lateral malleoli with abductor hallucis and abductor digiti minimi, respectively.
SELECTED NEOPLASMS OF THE SKIN
The following sections detail some of the soft tissue tumors that commonly affect the foot and ankle as well as several rare tumors of importance relative to the lower extremities.
BENIGN EPIDERMAL CELL TUMORS
Seborrheic Keratosis
Seborrheic keratosis, also known as basal cell papillomatosis, is a benign tumor that commonly occurs on the anterior leg and dorsum of the foot, and it is not found on the soles (Fig. 91.6). It is usually found in middle-aged and older individuals. The lesions are oval or round, flat or domed, verrucous, brown to black, papular, sharply demarcated, and display a stuck-on appearance. The papillomatous form is also referred to as stucco keratosis (see below) and may present in patches consisting of several lesions. A strong expression of the cyclindependent kinase inhibitor p27KIP1 appears to be the major mechanism of controlling the keratinocyte proliferation (4). The SK rarely transforms into a malignant basal or a squamous
cell carcinoma, and the standard mode of management entails observation or treatment by means of curettage or cryogenic destruction.
cell carcinoma, and the standard mode of management entails observation or treatment by means of curettage or cryogenic destruction.
Stucco Keratosis
Stucco keratosis is actually a variant of SK and is most commonly identified in the lower extremities in middle-aged to elderly persons. It presents clinically as tan to white papules with flat, hyperkeratotic surfaces. The surrounding skin is usually xerotic and scaly. Microscopically, parakeratosis mixed with orthokeratosis is observed. Treatment is usually not indicated; however, keratolytics, α-hydroxy acids, topical vitamin D3, curettage, or cryotherapy can be used if reduction of hyperkeratosis and hydration are desired.
Kyrle Hyperkeratosis
Kyrle hyperkeratosis presents as a crusty, hyperkeratotic, plug-like lesion that penetrates into the dermis and causes an inflammatory reaction with surrounding induration (5). The lesion usually occurs on the legs and the soles. Microscopically, perifollicular hyperkeratosis with an inflammatory infiltrate is observed. Treatment consists of curettage and ablation.
Clear Cell Acanthoma
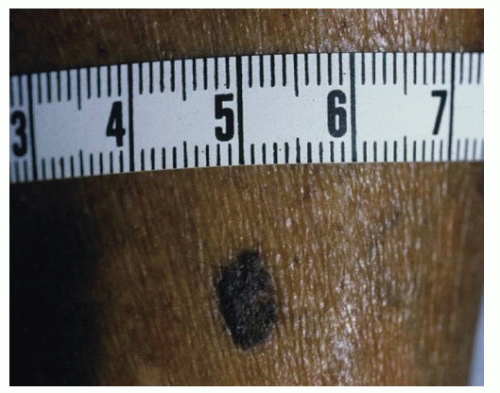
Clear cell acanthoma (CCA) presents as a sharply demarcated, solitary, red, crusty, or scaly, sometimes macerated and inflamed, nodule (Fig. 91.7). CCA usually occurs on the leg, and, like SK, it has a stuck-on appearance and is usually asymptomatic. Microscopically, CCA displays psoriasiform epidermal hyperplasia containing many keratinocytes, elongating and anastomosing rete ridges, and neutrophilic infiltrate (6). The periodic acid-Schiff stain is used for diagnosis and removal of staining after diastase digestion of the biopsy specimen, and treatment of the lesion is excision, cryotherapy (7), and/or curettage.
Epidermoid Inclusion Cyst
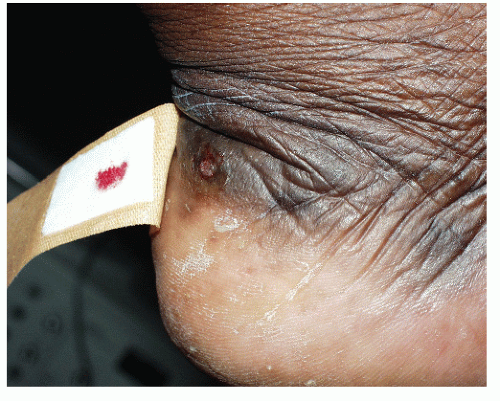
Epidermoid inclusion cyst (Fig. 91.8) usually occurs in young to middle-aged persons as single or multiple lesions that arise most commonly on the trunk, neck, and face. The lesion is precipitated by skin trauma, usually incidental (hence the high incidence of reported spontaneous origin), wherein epidermis is forced into underlying dermis and continues to desquamate and build up degenerated keratinocytes and keratin within the dermis. This process leads to the slow development of a firm, round, subcutaneous nodule. Pilar and sebaceous cysts are inclusion cysts that develop around the hair follicle. The tumor is usually 1 to 6 cm in diameter, and it is asymptomatic until the entrapped epidermal material becomes infected, usually by normal skin flora. Microscopically, the lesion displays intradermal cavities lined with epidermal cells and filled with serous fluid and degenerated epidermal cells. Treatment of this tumor is excision of the entire cyst.
PRECANCEROUS EPIDERMAL CELL TUMORS
Actinic Keratosis
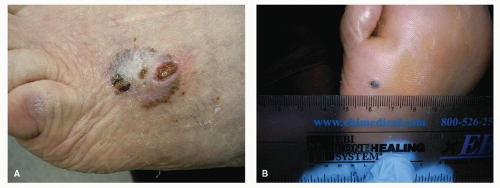
Actinic keratosis, also known as solar keratosis, occurs on sunexposed or, more commonly, sun-damaged skin surfaces in light-skinned persons (Fig. 91.9). Lesions can be multiple, and they frequently recur even after appropriate therapy. In the lower extremities, actinic keratosis most commonly localizes to the pretibial and dorsal surfaces. Actinic keratosis arises from within the epidermis and does not display a stuck-on appearance, thereby distinguishing this lesion from seborrheic and stucco keratoses. The lesions vary widely in diameter, from 1 to 2 mm up to 1 cm. Clinically, actinic keratosis displays hyperkeratosis, with dermal inflammation, telangiectasia, and elastosis. Up to 25% of actinic keratoses convert to carcinoma in situ and then progress to squamous cell carcinoma (8). Signs of malignant transformation include inflammation and the presence of a cutaneous horn. Treatment is with curettage and electrodessication or cryotherapy for isolated lesions, whereas multiple or widespread lesions are treated with 5-fluorouracil, dermabrasion, or topical photodynamic therapy with 5-aminolaevulinic acid (9).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree