CHAPTER 14 Sepsis of the Shoulder: Molecular Mechanisms and Pathogenesis
Shoulder sepsis can have a devastating impact on shoulder function, particularly if diagnosis and treatment are delayed or inadequate. The general principles in the pathogenesis of shoulder sepsis are similar to those pertaining to all intra-articular infections. There are three fundamental pathways for pathogens to enter a joint:
Certain patient groups with immune system depression or aberrations are at increased risk for infection. Patients with rheumatoid disease can manifest a spontaneous and somewhat cryptic sepsis in joints.1,2 Diabetics, infants, children, the aged, patients with vascular disease, drug abusers, and patients with HIV infection have an increased susceptibility to specific organisms, as are patients with hematologic dyscrasias and neoplastic disease.
Joint infection requires a threshold inoculum of bacteria and can be facilitated by damaged tissue, foreign body substrata, and the acellularity of cartilage surfaces. Total joint arthroplasties are at potential risk because of the presence of metal and polymer biomaterials and the decreased phagocytic ability of macrophages in the presence of methylmethacrylate. Biomaterials and adjacent damaged tissues and substrates are readily colonized by bacteria in a polysaccharide biofilm that is resistant to macrophage attack and antibiotic penetration.3,4 With antibiotic prophylaxis, published infection rates of total joint arthroplasty are low: 1% to 5%, depending on the device and the location.5,6 However, once infected, biomaterials and damaged tissues are exceedingly resistant to treatment.
Clinical infection in immunosuppressed patients involves the maturation of an inoculum of known pathogens (e.g., Staphylococcus aureus or Pseudomonas aeruginosa) or the transformation of nonpathogens (Staphylococcus epidermidis) to a septic focus of adhesive virulent organisms. This transformation can occur in the presence of, and be potentiated by, the surface of biomaterials,7,8 damaged tissue, and defenseless cartilage matrix surfaces.9
HISTORY
The latter part of the 19th century also saw the development of the concept of antisepsis. Lister maintained that sepsis was the main obstacle to significant advances in surgery. He documented a dramatic drop in cases of empyema, erysipelas, hospital gangrene, and surgical infection through the use of antiseptictechniques. Although the popularization of antiseptic technique in the surgical theater greatly reduced the rate of complications resulting from infection, it was not until the 1930s that specific antimicrobial therapy was discovered. In 1935, a German bacteriologist, Gerhard Domagk, discovered that sulfonamides protected mice against fatal doses of hemolytic staphylococci. Sulfonamides were soon employed for infections in patients, with excellent results.
Although the history of bacteriology, antiseptic techniques in surgery, and the development of antibiotics are well documented, very little of the early literature relates specifically to infections about the shoulder. In Codman’s book, The Shoulder, first published in 1934, infection of the shoulder and, in particular, osteomyelitis of the proximal humerus were considered very rare lesions.10 Codman cited a report by King and Holmes in 1927 in which a review of 450 consecutive symptomatic shoulders evaluated at the Massachusetts General Hospital revealed 5 cases of tuberculosis of the shoulder, 1 luetic infection of the shoulder, 3 unspecified shoulder infections, and 2 cases of osteomyelitis of the proximal humerus. The rarity of tubercular lesions of the shoulder was documented through the results of four large series of tuberculosis involving the musculoskeletal system (Townsend, 21 of 3244 cases; Whitman, 38 of 1833 cases; Young, 7 of 5680 cases; Billroth, 14 of 1900 cases). As microbial culturing and identification techniques developed in the early 20th century, streptococcal and staphylococcal species were more often identified as the causative agents in shoulder infection.
SEPTIC ANATOMY OF THE SHOULDER
Classically, the age-dependent presentations of hematogenous osteomyelitis and septic arthritis of the shoulder (and other large joints such as the hip and knee) have been attributed to the vascular development about the growth plate and epiphysis. The most detailed studies of the vascular development in this area have been done on the proximal femur but are analogous to the same development about the proximal humerus. Experimental work by Trueta11 demonstrated that before 8 months of age, there are direct vascular communications across the growth plate between the nutrient artery system and the epiphyseal ossicle. This observation was believed to account for the frequency of infection involving the epiphyseal ossicle and subsequent joint sepsis in infants. At some point between 8 months and 18 months of age (an average of 1 year), the growth plate forms a complete barrier to direct vascular communication between the metaphysis and epiphysis. The last vestiges of the nutrient artery turn down acutely at the growth plate and reach sinusoidal veins. At this point the blood flow slows down, creating an ideal medium for the proliferation of pathogenic bacteria.12
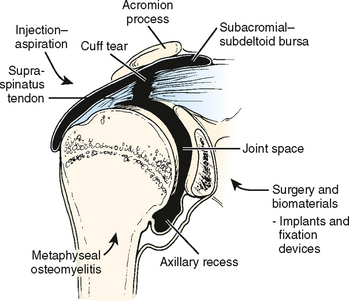
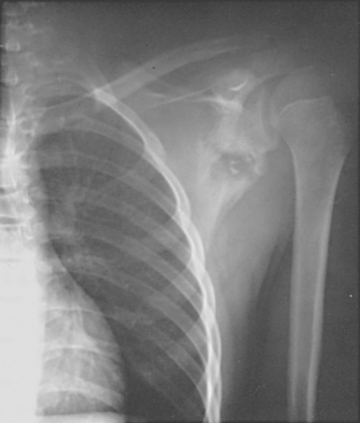
In the adult shoulder, the intra-articular extent of the metaphysis is located in the inferior sulcus and is intracapsular for approximately 10 to 12 mm.13 Infection of the proximal metaphysis, once established, can gain access to the shoulder joint via the haversian and Volkmann canals at the nonperiosteal zone (Fig. 14-2). With obliteration of the growth plate at skeletal maturity, anastomoses of the metaphyseal and epiphyseal circulation are again established.
In his study of the vascular development of the proximal femur, Chung did not find evidence of direct communications between the metaphyseal and epiphyseal circulation across the growth plate in any age group.14 Chung’s work demonstrated a persistent extraosseous anastomosis between metaphyseal and epiphyseal circulation on the surface of the perichondral ring. He found no evidence of vessels penetrating the growth plate in the infant population and attributed apparent changes in the arterial supply with age to enlargement of the neck and ossification center.
Branches of the suprascapular artery and the circumflex scapular branch of the subscapular artery from the scapular side of the shoulder anastomose with the anterior and posterior humeral circumflex arteries from the humeral side of the shoulder. This anastomotic system supplies the proximal humerus by forming an extra-articular and extracapsular arterial ring. Vessels from thisring traverse the capsule and form an intra-articular synovial ring. This fine anastomosis of vessels in the synovial membrane is located at the junction of the synovium and the articular cartilage. This subsynovial ring of vessels was first described by William Hunter in 1743 and named the circulus articuli vasculosus.15 At the transitional zone, synovial cells become flattened over this periarticular vascular fringe. Fine arterioles at this boundary acutely loop back toward the periphery. Again, blood flow at this level can decrease, which provides a site for establishing an inoculum of pathogenic organisms. Rather than hemodynamic changes, however, it is more probable that receptor-specific, microbe–to–cell surface interactions potentiate the infectious process.
Ward and Eckardt16 reported on four subacromial or subdeltoid bursa abscesses. Three of the four patients were chronically ill and debilitated, and in these patients the bursal abscesses coexisted with clinically diagnosed mild to resolving glenohumeral pyarthrosis. The symptoms and signs of these abscesses were minimal in all four patients. Ward and Eckardt found that computed tomography (CT) or magnetic resonance imaging (MRI) can help to detect abscesses and plan treatment.
MICROANATOMY AND CELL BIOLOGY
The hyaline cartilage of the articular surface is essentially acellular and consists of horizontally arranged collagen fibers in proteoglycan macromolecules. The boundaries of the joint cavity are composed of a richly vascularized cellular synovial tissue. It has been suggested that collagen fibers and the glycoprotein matrix, rather than the synovium, are the target substrata for microbial adhesion and colonization.9,17
Some synovial cells are phagocytic and appear to combat infection as part of the inflammatory response. Microscopic examination of infected joints in a lupine animal model indicated predominant colonization of cartilaginous, rather than synovial, surfaces.9 Receptors for collagen have been identified on the cell surfaces of certain strains of S. aureus.17 The infrequent occurrence of bacteria on synovial tissue might reflect innate resistance of synovial cells to colonization, the lack of appropriate synovial ligands, or functional host defense mechanisms at a synovial level.18 The subintimal vascularized layer contains fibroadipose tissue, lymphatic vessels, and nerves. Ultrastructural studies of the synovial subintimal vessels reveal that gaps between endothelial cells are bridged by a fine membrane. There is no epithelial tissue in the synovial lining and, therefore, no structural barrier (basement membrane) to prevent the spread of infection from synovial blood vessels to the joint. The synovial lining in the transition zone is rarely more than three or four cell layers thick, placing the synovial blood vessels in a superficial position. Intra-articular hemorrhage caused by trauma, combined with transient bacteremia, may be implicated as a factor in the pathogenesis of joint sepsis. Hematogenous seeding can allow bacterial penetration of synovial vessels, producing an effusion consisting primarily of neutrophils that release cartilage-destroying lysosomal enzymes.
Articular (hyaline) cartilage varies from 2 to 4 mm in thickness in the large joints of adults. This avascular, aneural tissue consists of a relatively small number of cells and chondrocytes and an abundant extracellular matrix. The extracellular matrix contains collagen and a ground substance composed of carbohydrate and noncollagenous protein and has a high water content. The chondrocytes are responsible for the synthesis and degradation of matrix components and are therefore ultimately responsible for the biomechanical and biologic properties of articular cartilage. Collagen produced by the chondrocytes accounts for more than half of the dry weight of adult articular cartilage (type II). Individual collagen fibers, with a characteristic periodicity of 640 Å, vary from 300 to 800 Å in diameter, depending on their distance from the articular surface.
Zone 1 contains large bundles of collagen fibers that are approximately 340 Å thick and lie parallel to the joint surface and at right angles to each other (Fig. 14-3). This zone, the lamina splendins, has little or no intervening ground substance and contains the highest density of collagen. Chondrocytes in zone 1 are ellipsoid and are oriented parallel to the articular surface. They show little electron microscopic evidence of metabolic activity.
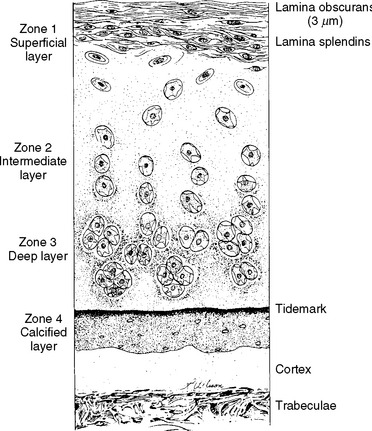
FIGURE 14-3 The zones of adult articular cartilage.
(Modified with permission from Turek SL: Orthopaedics: Principles and Their Application. Philadelphia: JB Lippincott, 1977.)
Bone is a composite structure incorporating calcium hydroxyapatite crystals in a collagen matrix grossly similar to synthetic composites or to partially crystalline polymers. Devitalized bone provides a passive substratum for bacterial colonization and the ultimate incorporation of its proteinaceous and mineral constituents as bacterial metabolites.3 S. aureus binds to bone sialoprotein, a glycoprotein found in joints, and it produces chondrocyte proteases that hydrolyze synovial tissue.19
CLASSIFICATION
Intra-articular sepsis may be classified in order of pathogenesis and frequency as direct hematogenous; secondary to contiguous spread from osteomyelitis; or secondary to trauma, surgery, or intra-articular injection (Fig. 14-4 and Box 14-1). Most joint infections are caused by hematogenous spread, although direct contamination is not uncommon with trauma. Inoculation of the joint with bacteria can occur in association with intra-articular injection of steroid, local anesthetic, or synthetic joint fluid. Infection rates following arthroscopy are also low, ranging from 0.4% to 3.4%.20 Armstrong and Bolding report that sepsis following arthroscopy can be associated with inadequate arthroscope disinfection and the use of intraoperative intra-articular corticosteroids.21
Osteomyelitis of the humerus can spread intra- articularly, depending on the age of the patient, the type of infecting organism, and the severity of infection. Osteomyelitis of the clavicle or scapula is uncommon, although it can occur after surgery and internal fixation, from retained shrapnel fragments, or in heroin addicts.22–25 Brancos and associates reported that S. aureus and P. aeruginosa were the etiologic agents in 75% and 11% of episodes, respectively, of septic arthritis in heroin addicts.26 The sternoclavicular joint was involved more commonly than the shoulder joint. Septic arthritis of the shoulder has been reported by Chaudhuri and associates following mastectomy and radiotherapy for breast carcinoma.27 Lymphedema was present in all cases. The infection had a subacute onset in all cases, and delays in diagnosis led to destruction of the joint in all but one patient.
Hematogenous osteomyelitis, although common in children,28 is uncommon in adults until the sixth decade or later and is usually associated with a compromised immune system. Intravenous (IV) drug use is associated with the development of osteomyelitis in adults. Direct spread from wounds or foreign bodies, including total joint and internal fixation devices, is the most common etiology of shoulder sepsis in adults. Gowans and Granieri noted the relationship between intra-articular injections of hydrocortisone acetate and the subsequent development of septic arthritis in 1959.29 Kelly and associates noted that two of their six patients had a history of multiple intra-articular injections of corticosteroid.30 Ward and Goldner reported that chronic disease was present in more than half of their 30 cases of glenohumeral pyarthrosis and that 4 cases were associated with ipsilateral forearm arteriovenous dialysis fistulas.31 Soft tissue infection about the shoulder can also manifest in the form of pyomyositis, sometimes occurring as a result of hematogenous spread.32 Aglas and associates reported sternoclavicular joint arthritis as a complication of subclavian venous catheterization.33 Their two cases responded to antibiotic treatment. Glenohumeral pyarthrosis has been observed following acupuncture.34 Lossos and coworkers reported that associated medical conditions were present in the majority of their patients with septic arthritis of the shoulder.35
PATHOGENIC MECHANISMS OF SEPTIC ARTHRITIS AND OSTEOMYELITIS
Surfaces as Substrata for Bacterial Colonization
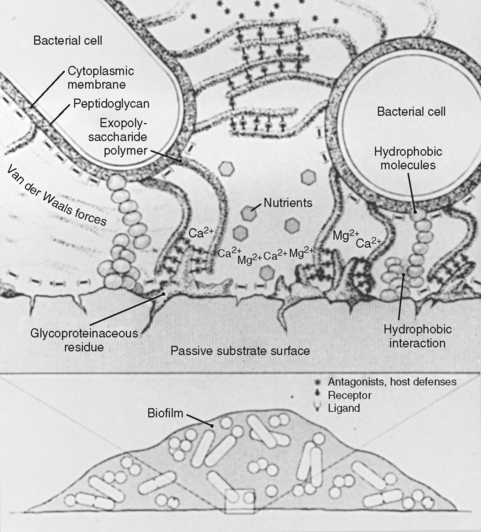
The pathogenesis of bone and joint infections is related, in part, to preferential adhesive colonization of inert substrata whose surfaces are not integrated with healthy tissue composed of living cells and intact extracellular polymers such as the articular surface of joints or damaged bone (Fig. 14-5).3,36–39 Almost all natural biologic surfaces are lined by a cellular epithelium or endothelium. The exceptions are intra-articular cartilage and the surface of teeth. Mature enamel is the only human tissue that is totally acellular; it is primarily composed of inorganic hydroxyapatite crystals (96% by weight), with a small amount of water (3%) and organic matrix (<1%).40 Proteins in the organic matrix are distributed between the hydroxyapatite crystals, forming a framework that strengthens the enamel by decreasing its tendency to fracture or separate. These proteins are unique among mineralized tissue because they are not a fibrous collagen protein like that found in bone, dentin, and cementum or in cartilage but are similar to the keratin family of proteins.
Enamel is mostly crystalline and inorganic, and cartilage is organic and noncrystalline. Enamel contains no collagen, whereas collagen is ubiquitous in cartilage and bone. S. aureus is the natural colonizer of cartilage but not of enamel, because it contains collagen receptors.41,42 The specificity of colonization is also modulated in part by lectins and the host-derived synovial fluid, blood, and serum; conditioning films of protein; and polysaccharide macromolecules. The colonization of teeth by Streptococcus mutans and other organisms is a natural polymicrobial process and may be symbiotic or slowly destructive, but bacterial colonization of articular cartilage is unnatural and rapidly destructive.
The acellular cartilage matrix and inanimate biomaterial surfaces offer no resistance to colonization by S. aureus and S. epidermidis, respectively. The observed invasion and gradual destruction of the cartilage over time supports clinical observations of the course of untreated septic arthritis (Fig. 14-6).43,44 The acellular cartilaginous surfaces of joints are particularly vulnerable to sepsis because they allow direct exposure to bacteria from open trauma, surgical procedures, or hematogenous spread. This mechanism parallels previous observations on the mechanism of osteomyelitis, which suggest that the adhesion of bacteria to dead bone or cartilage (surfaces not protected by living cells) via receptors and extracellular polysaccharides is a factor in pathogenesis.3,45
Intra-articular Sepsis
Spontaneous intra-articular sepsis of the shoulder is derived from random hematogenous bacterial seeding. The synovial vasculature is abundant, and the vessels lack a limiting basement membrane. Bacteremia, especially Neisseria gonorrhoeae, increases the risk of intra-articular spread. Nosocomial infections in neonatal intensive care units are usually related to the presence of intravascular devices.46 Contiguous spread from adjacent and intracapsular metaphyseal osteomyelitis also occurs. Surgery, arthroscopy, total joint replacement, aspirations, and steroid injections also can result in direct inoculation of bacteria into the intra-articular space. The presence of foreign bodies from trauma or after surgery (stainless steel, chrome-cobalt alloys, ultra-high molecular weight polyethylene, and methylmethacrylate) increases the possibility of infection by providing a foreign body nidus for colonization, allowing antibiotic-resistant colonization to occur.3,7 The presence of a foreign body also decreases the size of the inoculant required for sepsis and perturbs host defense mechanisms.
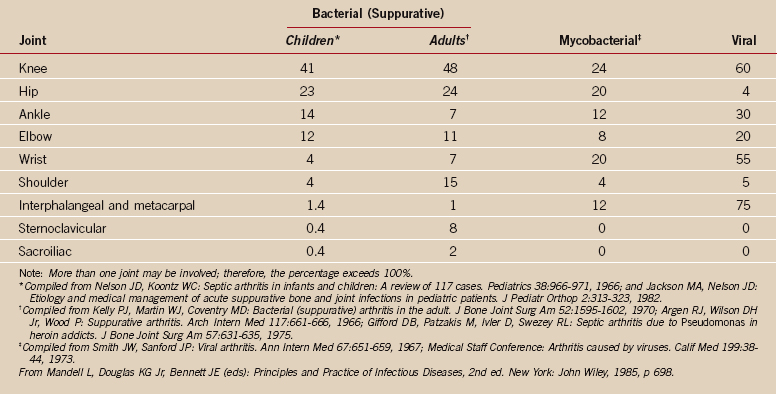
Septic arthritis of the shoulder represents up to 14% of all septic arthritis cases.47 In earlier studies, the incidence was 3.4%.48 Caksen and associates report shoulder involvement in 2 of 49 joints involved in their series of pediatric patients with septic arthritis.49 A more elderly population, increased trauma, and the common use of articular and periarticular steroids may be factors in this epidemiology (Fig. 14-7). The primary causal organism of shoulder sepsis is S. aureus. Sepsis in immunocompromised patients may be polyarticular as well as polymicrobial. Ten percent of septic arthritis involves more than one joint and is likely to occur in children.
Osteomyelitis
Hematogenous osteomyelitis accounts for most cases of osteomyelitis in children. Contiguous osteomyelitis is more common in adults, secondary to surgery and direct inoculation. In persons older than 50 years, contiguous osteomyelitis and disease related to vascular insufficiency are predominant. Of the bones of the shoulder, the humerus is most commonly involved in osteomyelitis. The clavicle is occasionally involved in drug addicts by hematogenous spread. The scapula is rarely involved; usually infection occurs by direct inoculation or contiguous spread (Table 14-1).
Epps and associates reviewed 15 patients who had sickle cell disease and osteomyelitis affecting 30 bones.50 S. aureus was isolated on culture of specimens of bone from 8 of the 15 patients; Salmonella species from 6; and Proteus mirabilis from 1. Accordingly it appears that Salmonella species are not always the principle causative organisms of osteomyelitis in patients who have sickle cell disease.
MICROBIAL ADHESION AND INTRA-ARTICULAR SEPSIS
An understanding of microbial adhesion is required for complete clinical and therapeutic insights in joint sepsis. Studies of bacteria in marine ecosystems indicate that they tend to adhere in colonies to surfaces or substrata. The number of bacteria that can exist in a given environment is related directly to stress and nutrient supply.51 Because surface attachment, rather than a floating or suspension population, is a favored survival strategy, it is the state of the major portion of bacterial biomasses in most natural environments and is a common mode of microbial life in humans.
Bacterial attachment to surfaces is influenced by proteinaceous bacterial receptors and by an extracapsular exopolysaccharide substance within which bacteria aggregate and multiply.52 Once bacteria have developed a biofilm-enclosed adhesive mode of growth, they become more resistant to antiseptics,53 antibiotics,54 and host defense systems.39,54,55 Free-floating, nonadhesive bacteria or microbes that lack a well-developed outer layer or exopolysaccharide are more susceptible to host-clearing mechanisms56 and to lower concentrations of antibacterial agents.54 Gibbons and van Houte first described the significance of this adhesive phenomenon in the formation of dental plaque.57 In diseases such as gonococcal urethritis,58 cystic fibrosis,59 and endocarditis, bacterial colonization and propagation occur along endothelial and epithelial surfaces. The association between bacterial growth on biomaterial surfaces and infection was first described in 1963.60
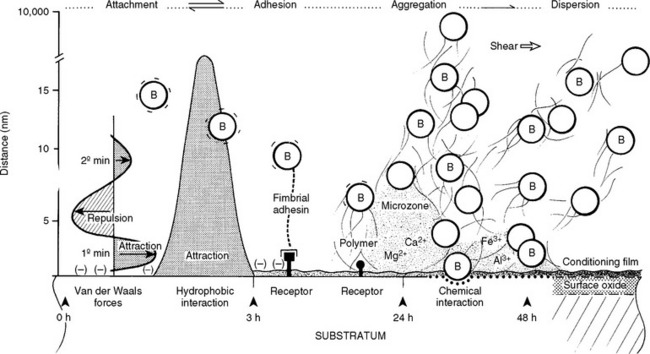
Initial bacterial attachment or adhesion depends on the long-range physical force characteristics of the bacterium, the fluid interface, and the substratum. Specific irreversible adhesion, which occurs after initial attachment, is based on time-dependent adhesin–receptor interactions and on extracapsular polysaccharide synthesis.61 Biomaterial surfaces present sites for environmental interactions derived from their atomic structures (Fig. 14-8).62 Metal alloys have a thin (100-200 Å) oxide layer that is the true biological interface.63,64 The surfaces of polymers and metals are modified by texture, manufacturing processes, trace chemicals, and debris and also by host-derived ionic, polysaccharide, and glycoproteinaceous constituents (conditioning films). The finite surface structure of conditioning film in a human is specific for each individual biomaterial, type of tissue cell, and local host environment.65 Tissue cells and matrix macromolecules also provide substrata for bacterial colonization. Bacteria have developed adhesins or receptors that interact with tissue cell surface structures (Fig. 14-9).
Subsequent to or concomitant with initial attachment, fimbrial adhesins (E. coli) and substratum receptors can interact, as in bacteria–to–tissue cell pathogenesis or for the glycoproteinaceous conditioning films that immediately coat implants.65 The production and composition of the extracellular polysaccharide polymer, which tends to act like a glue, is a pivotal factor.51 After colony maturation, cells on the periphery of the expanding biomass can separate or disaggregate and disperse, a process that is moderated by colony size, nutrient conditions, and hemodynamic or mechanical shear forces. In natural environments, disaggregation is a survival strategy; in humans, however, it is involved in the pathogenesis of septic emboli. Disaggregation (dispersion) and its parameters might explain the phenomenon of intermittent or short-term bacterial showers or disseminated bacterial emboli.
BACTERIAL PATHOGENS
Organisms involved in septic arthritis and osteomyelitis of the shoulder (in order of frequency)30 include S. aureus, S. epidermidis, Streptococcus group B, E. coli, P. aeruginosa, Haemophilus influenzae type B, N. gonorrhoeae, Mycobacterium tuberculosis, Salmonella and Pneumococcus species, and Yersinia enterocolitica.66 Reported associations67–72 and other etiologic pathogens are listed in Box 14-2.