Selected Tendon Transfers for Median, Ulnar, and Radial Nerve Deficits
Allan E. Peljovich
Benjamin J. Rogozinski
Michael W. Keith
INTRODUCTION
Peripheral nerve injuries of the upper extremities will leave individuals with real impairments. These impairments tend to follow predictable patterns determined by the particular nerve(s) affected, the specific anatomic location of the damage, and the extent of concomitant injuries to bone and soft-tissue structures. Fortunately, these impairments can be mitigated or overcome by sophisticated orthotics/physiotherapy, individual compensation, and operations designed to address some of the specific impairments. In the acute and subacute setting, elegant techniques to repair and reconstruct damaged nerves are favored. Tendon transfers are typically performed when nerve recovery is deemed impossible by the proximal location of an injury or a particularly large zone of damage, when nerve reconstructions and/or transfers are contraindicated or have failed to result insufficient reinnervation of paralyzed muscles, or even early following injury to minimize bracing (wrist extension transfer and radial nerve palsy). This chapter discusses general concepts in thinking about and executing tendon transfers and then illustrates these concepts and surgical techniques as they apply to median, ulnar, and radial nerve deficits.
GENERAL INDICATIONS
Tendon transfer surgery is founded on the anatomical duplicity present in our upper extremities, that is, multiple muscles mobilizing one joint. On the other hand, these techniques are limited by the inability to fully reconcile all losses from paralysis. Donor muscles are not the biomechanical equivalent of the recipient muscles they are “replacing” and come at some cost in terms of losing the original function of the donor. General indications are outlined below:
Timing The individual’s functional disabilities should be considered permanent. Operative techniques that could restore peripheral nerve integrity should be considered to have precedence over tendon transfers, unless there are extenuating circumstances that necessitate earlier functional improvement and the individual is willing to make this choice. The anatomical environment should also be stable. Wounds should be fully healed, any fractures should be united, and tissues should generally be in a state of equilibrium.
Splinting/adaptations insufficient A variety of orthotics exist to help compensate for some of the function lost by nerve palsies. Anticlawing splints for ulnar nerve palsy, soft opponens splints for median nerve palsies, and metacarpophalangeal (MCP) extension splints for posterior interosseous nerve (PIN) palsy should be entertained and trialed in individuals. Some individuals will decide that these modifications are sufficient, and they seek no further treatment. Many will conclude that the braces are cumbersome, are tiresome, and/or fail to produce a desired improvement, and surgery becomes an appropriate option for them.
Availability of appropriate donor muscles
Surgery should not sacrifice important present function in one joint to improve lost function in another.
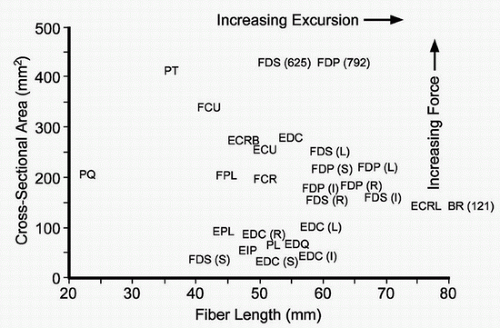
The donor muscle should be biomechanically similar, and nearby, to the recipient muscle. For example, the pronator teres (PT) might serve as a poor replacement for the flexor digitorum profundus (FDP if the surgeon’s goal is full finger flexion given its relative lack of excursion. Beware of using a donor muscle that is weaker than the recipient if strength of restoration is important (Fig. 17-1).
The donor muscle should be relatively “intact,” that is, strong, free of injury, free of spasticity, and under the individual’s volitional control.
Availability of appropriate recipients
The surgeon should think in terms of lost functions, not muscles. For example, median nerve injury results in loss of thumb opposition, not loss of the 3 thenar intrinsic muscles that contribute to opposition. The limited nature of muscle redundancy requires the surgeon to perform an operation that restores the lost function as precisely as possible given he/she will not be able to replace the loss of every paralyzed muscle.
The recipient joints need to be supple. Tendon transfers do not treat stiffness.
Healthy tissue “bridge” Tendon transfers involve reorienting a muscle/tendon to a nearby, but new, insertion. The tissue bridge between the donor and recipient should allow for excursion. In the setting of significant trauma, tissue bridges made of skin graft devoid of subcutaneous fat and filled with previously placed internal hardware, even when fully healed, may create an environment that promotes adhesions along the route of the tendon transfer, limiting excursion.
Availability of resources Successful tendon transfer operations require frequent physiotherapy and frequent office visits over the course of a few months. Individuals unable to manage both are unlikely to succeed.
Appropriate motivation “Learning” and incorporating tendon transfers into daily life requires individuals who are committed and ready for this process. Good surgeon-individual communication is required to ensure that an individual’s needs are understood, the surgical expectations are appropriate (not excessive), and the goals are realistically achievable.
CONTRAINDICATIONS
The reasons not to proceed with tendon transfer restoration in the setting of nerve palsies are similar regardless of the actual palsy.
Individuals who do not perceive disability and feel “adapted” to their circumstance.
Insufficient time has passed from nerve insult to conclude the individual’s physical state as a permanent one or one that cannot be altered by other means.
An individual’s physical examination, that is, degree of paralysis, recipient joint contractures, loss of tissue, or state of tissue health, eliminates the realistic possibility of a successful tendon transfer procedure.
Individuals who lack the resources necessary to effect postoperative therapy.
Individuals who are not committed to the recovery process and therapy, in particular.
Individuals with greater than realistic expectations are a relative contraindication.
PREOPERATIVE PREPARATION
Developing a surgical plan follows a fairly routine algorithm. Adjustments and creativity are occasionally needed in order to apply “standard” sets of tendon transfers to a particular individual’s situation, that is, soft-tissue damage compromises standard donor muscles or recipient tendons. The importance of good two-way communication between the surgeon and the individual allows the creation of a sound strategy that will help solve the individual’s particular needs.
Identify the lost critical hand functions. What, exactly, needs restoration? The focus is on function as it relates to how hands manipulate their environment and not the various muscles themselves. Low median nerve damage compromises large object manipulation via loss of opposition. Ulnar nerve damage leads to multiple impairments including compromised lateral pinch, inefficient finger flexion and clawing, and small finger abduction, but not every functional loss requires reconstruction when there are limited options. Good surgeon-individual communication is important here so as not to reconstruct elements of paralysis that do not require treatment.
Distill each lost function into as few recipient muscles as possible. Restoration of finger flexion requires only restoration of the FDP.
Identify available and biomechanically similar donor muscles to replace the lost recipient “function.” The surgeon should review various alternative donor muscles (1) (Fig. 17-1).
The individual must be able to voluntarily contract the potential donor muscle.
The potential donor should have sufficient strength (BMRC ≥4).
Force of contraction should be sufficient for the lost function.
Muscle excursion (shortening) should produce sufficient functional motion.
Loss of the donor muscle’s native function should not create real disability.
In-phase donor muscles are ideal and preferred, but not always necessary. Individuals who can learn to volitionally contract the donor muscle will be able to learn the transfer, but it is substantially easier to incorporate into their function when the donor muscle is already in phase to the lost function, that is, wrist flexion and finger extension.
Ensure joint suppleness of the recipient “functions.” Early splinting and physiotherapy can maintain joints following injuries, and surgery is sometimes required to release contractures prior to tendon transfer surgery.
Consider lost sensation from the nerve injury and how this could influence the decision to operate and the result of surgery. All individuals are different and their “need” for good sensate skin in the operative plan. This is a particular issue with median nerve palsies.
TECHNIQUE
Plan surgical approaches. Access to the donor tendon insertion and the access to mobilize the donor can be through separate small approaches or a larger approach. Access to the recipient tendons may require a separate exposure. Coaptation to restore extrinsic forearm flexors and extensor function should be placed proximal to the carpal tunnel and extensor retinaculum whenever possible to avoid adhesions.
Mobilize the donor muscle. The force of donor muscle contraction can be diminished with the tendon sets off at an angle to the body of the muscle and the orientation of its sarcomeres. While some transfers require vector changes, most will do best when the muscle contraction is parallel to the tendon to maximize force. This may require several small exposures or one larger exposure. The brachioradialis (BR) and the flexor carpi ulnaris (FCU) are two examples of muscles with broad attachments that require mobilization (2,3).
Avoid creating new areas of compression or friction. As the donor tendon is mobilized to a new area, the surgeon will think about its orientation with respect to other muscles, sensory nerves, and blood vessels.
Recipient considerations. Bear in mind that the excursion that results at the coaptation site could create sources of impingement or friction. This is particularly the case in transfers to restore the extrinsic extensor and flexor tendons where they run under the extensor and flexor retinaculum, respectively.
Setting tension
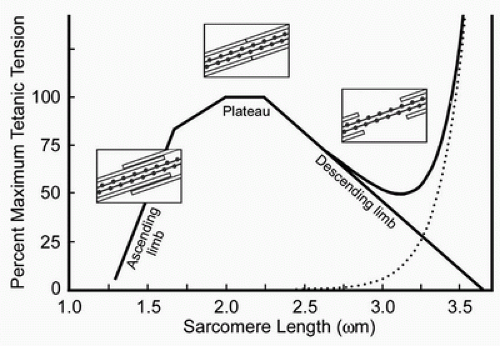
Tendon transfer tension should be ideally set at the resting length of the donor muscle to maximize its potential force of contraction (4). Setting transfers tightly simply put the muscle into the passive tension portion of its Blix curve and create an inefficient donor muscle (5,6) (Fig. 17-2). The difficult part is determining resting length, due to the lack of specialized equipment not currently commercially available (7,8,9,10,11). The authors currently estimate the resting length by allowing the donor muscle to rest at its natural tension prior to securing the coaptation. Hemostats placed on the donor tendon distal to the weave allow the surgeon an opportunity to test the tension using proximal joint passive tenodesis prior to formally securing the coaptation with sutures.
The position of the joint that mobilized via tendon transfer merits consideration as well. The resting length of the donor muscle should be set about the midrange of functional motion of the “recipient” joint (12). Tensioning at one end of motion will result in a transfer too loose at the opposite endpoint of range (Fig. 17-2).
Tendon coaptation. The Pulvertaft weave continues to be a mainstay of transferring a donor tendon into a recipient with strength and security, but is bulky (13,14). A variety of other techniques with biomechanical robustness have also been reported including the “spiral linking technique,” the “double loop technique,” and newer side-to-side repairs (30 to 34 JHS) (15,16,17,18,19) (Fig. 17-3).
Vector considerations. The traditional teaching is a straight line of pull from donor muscle to recipient insertion/transfer in order to minimize loss of contraction force from changes in angles. This line of pull, or vector, can be altered to create particularly desirable effects (20). In the case of opponensplasties, the surgeon can set the recipient thumb carpometacarpal motion from a spectrum of palmar abduction to circumduction to adduction depending upon whether the transfer “pulls” from the radial side of the wrist to the ulnar side and even distally from the hook of hamate (21,22). The surgeon can control how the transfer will work and adjust the procedure to the patient’s particular needs.
POSTOPERATIVE MANAGEMENT
Postoperative protocols call for a period of immobilization to protect the coaptation for about 3 weeks, followed by mobilization with splints and then transitioning to splint-free motion after about 6 to 8 weeks from surgery. Once mobilization starts, individuals are taught important techniques using biofeedback to learn how to fire their donor muscles to produce the desired recipient functions, and this is why in-phase donor muscles are easier to rehabilitate. It is important for individuals to be motivated to continue working their transfers long after formal therapy is concluded to help promote the neural learning required to make these transfers second nature.
MEDIAN NERVE PALSY
Indications
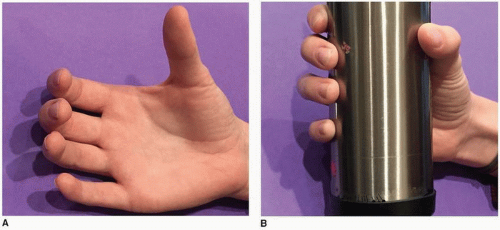
Median nerve paralysis tends to fall into two categories depending upon the anatomic location of the insult. Low median nerve damage refers to injury distal to the extrinsic flexor motor points and affects the thenar intrinsics and sensory innervation to the thumb/index/long/partial ring. The primary functional motor deficit is the lack of thumb opposition (ulnar intrinsics prevent clawing of the index and long), and the primary sensory deficit includes numbness to the thumb, index, long, and radial-sided ring fingers (Fig. 17-4). Individuals seeking restoration should demonstrate difficulties with large object acquisition and lateral pinch. Some can accommodate depending upon hand dominance, age, vocation, avocations, etc. High median nerve damage occurs more proximally in the forearm and additionally affects some combination of the flexor carpi radialis and palmaris longus (PL), the extrinsic finger flexors (except FDP to small and ring), the forearm pronators, and the flexor pollicis longus (FPL). Sensory loss to the base of the thenar eminence typically exists in high median nerve damage and can even occur in low damage provided the insult is just distal to the last flexor digitorum superficialis (FDS) motor branch. This more disabling presentation creates global problems with pinch and grasp, and individuals will nearly always seek options for improvement. The surgeon should never overlook the problems associated with sensory deficits to the radial sided digits, and should temper and/or incorporate how this affects the individual when rendering advice and formulating a surgical plan.
Preoperative Preparation
Tendon transfer for low median nerve palsy is geared toward restoring thumb opposition, hence large object acquisition. This motion is one of circumduction, a combination of palmar abduction and pronation (Fig. 17-5). Opposition is typically a positional function placing the thumb in a position to pinch and/or manipulate a larger object. Strength of pinch and manipulation are achieved via the ulnar innervated thenar intrinsics (adductor pollicis [AdP], flexor pollicis brevis [FPB]) and the FPL. Even in cases of high median palsy, the ulnar-innervated AdP and FPB can create sufficient force for pinch. Donor muscles therefore need to be strong enough to mimic the abductor pollicis brevis (APB) and shorten enough to create thumb circumduction. A variety of donor muscles have been utilized to restore opposition: FDS of the long or ring finger (not available in high median nerve palsy), extensor indicis proprius (EIP), extensor pollicis longus (EPL), extensor carpi ulnaris (ECU), extensor carpi radialis longus (ECRL), extensor digiti quinti (EDQ), PL, and abductor digiti minimi (ADM) (21,23,24,25,26,27,28,29,30,31,32,33,34,35,36). A still utilized classic technique to restore opposition was described by Bunnell using a donor muscle/tendon moving through a constructed pulley at the level of the pisiform, across the palm, and to the dorsal ulnar aspect of the thumb metacarpal (37).
The additional impairments associated with high median nerve palsy depend upon the location of the damage with respect to the nerve’s various branches in the forearm. These impairments may include loss of index and long finger flexion (loss of both FDP and FDS), weakening of ring and small flexion (loss of FDS), loss of forceful forearm pronation (PT and pronator quadratus [PQ]), and loss of thumb interphalangeal flexion (loss of the FPL). The loss of radial-sided flexion contributes to significant disability via the loss of various pinch patterns and the weakening of palmar grasp. Preoperative assessment will determine the extent to which any loss of forceful pronation is a real issue with the individual and will depend upon their vocations and avocations given that shoulder abduction and BR contraction will produce a forearm pronation moment. The added loss of all the FDS muscles necessitates alternative donor muscle for opposition among the potential donors previously listed. The EIP and ADM are common donor options in this setting. The FPL is often restored using the BR as the donor muscle, and index and long flexion are reanimated using side-to-side transfers of the ulnar-innervated FDP (28,38). In circumstances where independent index or index/long flexion is needed, the ECRL can be used as a donor motor for the radial-sided FDP muscles (28,39,40).
Two anatomical variations in forearm nerve anatomy between the median and ulnar nerves can affect the individual’s pattern of presentation. Both uncommon, the Martin-Gruber and Riche-Cannieu interconnections describe anomalous nerve crossovers that can lead to more or less paralysis than expected for median and ulnar nerve insults (41,42,43,44). In the Martin-Gruber anastomosis, ulnar intrinsic nerve fascicles are carried by the median nerve, often as part of its anterior interosseous
branch, into the target muscles in the hand causing a high median nerve palsy to present more like a combined median-ulnar nerve palsy in the hand. In the case of the Riche-Cannieu anastomosis, thenar intrinsic nerve fascicles are carried into the hand with the ulnar nerve; thus, individuals with median nerve damage present with retained thenar intrinsic muscle function. Electrophysiologic studies can help elucidate such interconnections when presented with an individual with a confusing presentation.
branch, into the target muscles in the hand causing a high median nerve palsy to present more like a combined median-ulnar nerve palsy in the hand. In the case of the Riche-Cannieu anastomosis, thenar intrinsic nerve fascicles are carried into the hand with the ulnar nerve; thus, individuals with median nerve damage present with retained thenar intrinsic muscle function. Electrophysiologic studies can help elucidate such interconnections when presented with an individual with a confusing presentation.
Technique
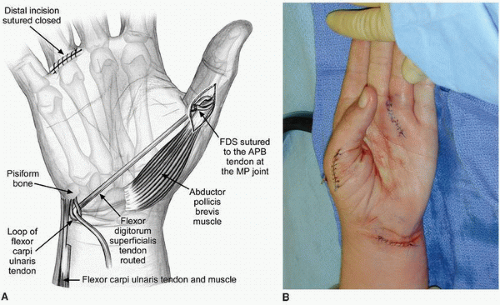
Low Median Nerve Palsy: Opponensplasty (Fig. 17-6A-G) The described technique uses the ring finger FDS as the donor motor, the APB tendon as the recipient, and a vector of force from the level of the pisiform to create the circumduction moment (21,22,27,45).
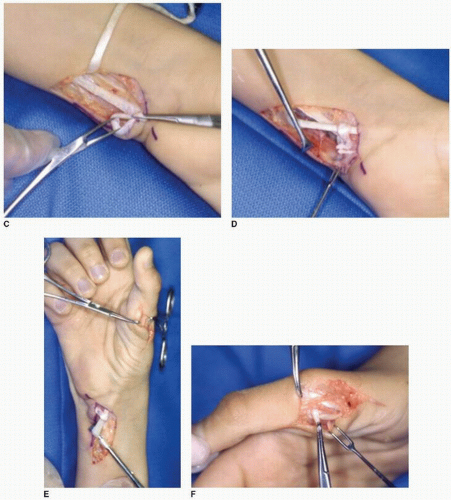
An oblique incision is made over the ring finger A1 pulley between the distal palmar and MCP joint creases (Fig. 17-6A,B). The A1 pulley is incised longitudinally, and the FDS tendon is isolated and separated from the FDP tendon (Fig. 17-6C). The ring finger is flexed, and the FDS tendon is divided transversely distal to its bifurcation. The tails of the FDS tendon inserting onto the middle phalanx are left behind to adhere to the floor of the tendon sheath and prevent PIP joint hyperextension.
A second longitudinal incision is now made along the radial margin of the thumb MCP joint long enough to expose the desired insertion site(s) (Fig. 17-6B). In most cases, where radial innervation is intact, the APB insertion will be the recipient. In some cases, where there may be need for simultaneous thumb MCP extension, an additional recipient will be the extensor pollicis brevis insertion/dorsal MCP capsule. The surgeon should mind the dorsal radial sensory nerve to the thumb in this exposure.
A third incision around the wrist creases is made in the region of the FCU tendon insertion into the pisiform to create the vector of pull (Fig. 17-6B). The FCU and the ring finger FDS tendons are exposed while ulnar neurovascular structures are protected. The radial half of the FCU tendon is divided transversely approximately 4 cm proximal to its insertion on the pisiform and mobilized creating a distally based tendon strip (Fig. 17-6D). This FCU tendon strip is looped distally and passed through the distal portion of the FCU near the pisiform insertion and secured with nonabsorbable
sutures, thus creating a new pulley for the donor muscle (Fig. 17-6D). The ring finger FDS tendon is identified in the same volar/ulnar wound, pulled into the proximal wound, and “threaded” through the FCU loop (Fig. 17-6E). A subcutaneous tunnel is created between this wound and the radial thumb wound superficial to the palmar aponeurosis (Fig. 17-6F). The donor tendon is routed through the pulley and into the radial thumb incision.
sutures, thus creating a new pulley for the donor muscle (Fig. 17-6D). The ring finger FDS tendon is identified in the same volar/ulnar wound, pulled into the proximal wound, and “threaded” through the FCU loop (Fig. 17-6E). A subcutaneous tunnel is created between this wound and the radial thumb wound superficial to the palmar aponeurosis (Fig. 17-6F). The donor tendon is routed through the pulley and into the radial thumb incision.
Several options for attachment of the tendon transfer have been described with more dorsal insertions enhancing pronation and more radial attachments yielding greater abduction (31,46). The exact position for insertion depends upon the needs of the patient and his or her thumb determined prior to surgery, but is typically the tendon of the APB (Fig. 17-6G).
Regardless of the chosen insertion site, correct tensioning is imperative to achieve an optimal result. Tensioning is set with the wrist in neutral to slight extension and the thumb resting against the lateral aspect of the radial side of the index finger about its distal interphalangeal (DIP) joint. The estimated resting length of the donor is based upon its natural elasticity, allowing it to settle in its resting position. The result should be enhanced opposition with progressive wrist extension and relaxation as the wrist flexes beyond neutral. After skin closure, the thumb is immobilized in opposition and the wrist in slight extension keeping the coaptation without tension while maintaining comfort for the individual.
High Median Nerve Palsy
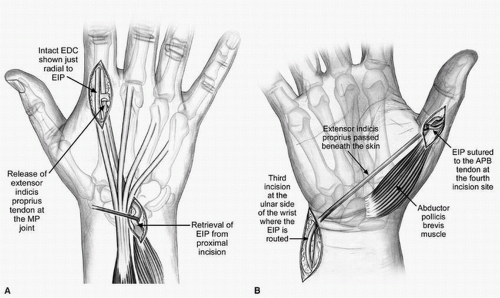
OPPONENSPLASTY USING EIP (FIG. 17-7A,B) The EIP as a donor motor was initially described by Burkhalter and is a fairly synergistic transfer (23,26). Although the EIP does not have the force of contraction of the FDS ring, it is still greater than that of the APB and can serve as an appropriate donor muscle. This procedure involves routing the EIP from its normal insertion into the extensor hood of the index MCP joint around the ulnar side of the wrist toward the APB insertion in the thumb.
The EIP is first harvested via a small approach in line with Langer’s lines at the level of the index extensor hood (Fig. 17-7A). The EIP is taken with some of the hood to maximize length, and the hood is repaired with nonabsorbable suture. A second longitudinal incision is created proximal to
the extensor retinaculum and more toward the ulnar side of the forearm where the EIP is identified and mobilized into (Fig. 17-7B). The distal muscle belly within the tendons of the fourth extensor compartment often identifies the EIP. It is mobilized from soft-tissue attachments to create straight line of pull from its origin. A third oblique or zigzag incision is now created along the palmar ulnar base of the wrist in the vicinity of the pisiform, and the EIP is drawn into this wound via a subcutaneous tunnel from the second exposure (Fig. 17-7B). Finally, a radial midaxial incision is created along the thumb MCP joint, and the APB insertion is identified while protecting the dorsal radial sensory nerve. A tunnel is created superficial to the palmar aponeurosis, and the EIP is brought into the thumb incision and coapted into the APB (Fig. 17-7B). Tension is set with the wrist in 10 degrees of extension, the thumb opposed against the side of the index finger, and the EIP in its natural elastic resting position. This position is the position of splinting/casting and may include other fingers depending upon other simultaneous transfers performed.
the extensor retinaculum and more toward the ulnar side of the forearm where the EIP is identified and mobilized into (Fig. 17-7B). The distal muscle belly within the tendons of the fourth extensor compartment often identifies the EIP. It is mobilized from soft-tissue attachments to create straight line of pull from its origin. A third oblique or zigzag incision is now created along the palmar ulnar base of the wrist in the vicinity of the pisiform, and the EIP is drawn into this wound via a subcutaneous tunnel from the second exposure (Fig. 17-7B). Finally, a radial midaxial incision is created along the thumb MCP joint, and the APB insertion is identified while protecting the dorsal radial sensory nerve. A tunnel is created superficial to the palmar aponeurosis, and the EIP is brought into the thumb incision and coapted into the APB (Fig. 17-7B). Tension is set with the wrist in 10 degrees of extension, the thumb opposed against the side of the index finger, and the EIP in its natural elastic resting position. This position is the position of splinting/casting and may include other fingers depending upon other simultaneous transfers performed.
FPL RESTORATION USING THE BRACHIORADIALIS (FIG. 17-8A-D) Utilization of the BR for FPL restoration has a long history (47,48). The BR is a secondary elbow flexor that is a very useful donor muscle provided the surgeon understands that much of its potential excursion is tied up in intermuscular fascial connections throughout the forearm; therefore, the surgeon must be prepared
to plan surgical approaches that allow for its more extensive mobilization requirements despite its tendon’s very close proximity to the FPL tendon in the forearm (2,32,49). The BR also possesses excellent potential contraction force, and so it is very suitable to help restore lateral pinch strength (50).
to plan surgical approaches that allow for its more extensive mobilization requirements despite its tendon’s very close proximity to the FPL tendon in the forearm (2,32,49). The BR also possesses excellent potential contraction force, and so it is very suitable to help restore lateral pinch strength (50).
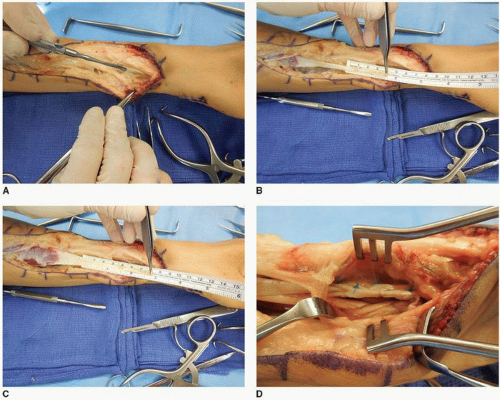 FIGURE 17-8 These clinical photographs demonstrate the salient features of the BR to FPL transfer. A:
Get Clinical Tree app for offline access
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|