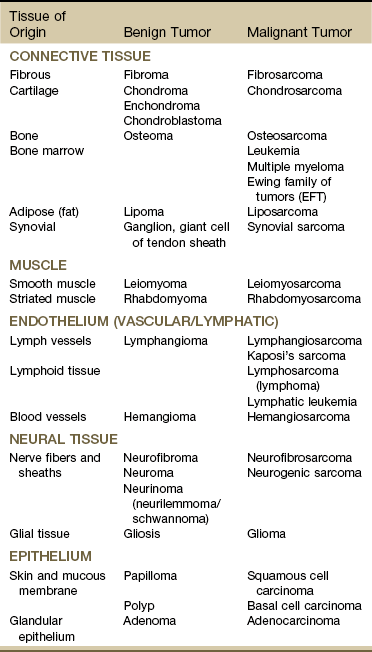
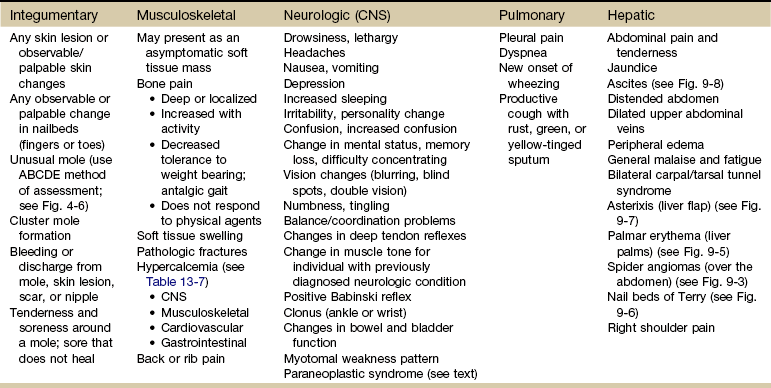
Chapter 13 • What questions would be appropriate for your first physical therapy interview with this client? • What test procedures will you carry out during the first session? • If you suggest to this man that he should see his physician, how would you make that recommendation? (See the Case Study at the end of the chapter.) A large part of the screening process is identifying red flag histories and red flag signs and symptoms. Advancing age and previous history of any kind of cancer are two of the most important risk factors for cancer. Following the screening model presented in Chapters 1 and 2, the therapist will use past medical history, clinical presentation, and associated signs and symptoms as the basic tools to screen for cancer. Cancer accounts for more deaths than heart disease in the United States in persons under the age of 85 years. There are more than 1.5 million new cases of cancer in the United States each year; more than half a million will die from cancer this year. One in four deaths in the United States is attributed to cancer.1 Predicting lifetime risk of cancer is based on present rates of cancer. Using today’s epidemiologic data, 44% of all men and 38% of all women will develop cancer at some time in their lifetime.1 It is estimated that by 2030, 20% of the U.S. population will be 65 years old or older, accounting for 70% of all cancers and 85% of all cancer-related mortality.2 In the past, certain types of cancer were invariably fatal. Today, however, death rates continue to decline for most cancers, and there continues to be a reported reduced mortality from cancer. The percentage of people who have survived longer than 5 years after cancer diagnosis has increased over the past 2 decades.3 Fig. 13-1 summarizes current U.S. figures for cancer incidence and deaths by site and sex. Although prostate and breast cancers are the most common malignancies in men and women, respectively, the cancer that most commonly causes death is lung cancer.1 Fig. 13-1 Estimated new cases of cancer and cancer deaths by site for men and women. Carcinoma in situ is not included in the statistics related to invasive carcinoma or sarcoma as reported by the American Cancer Society (ACS) or the National Cancer Institute (NCI). Carcinoma in situ is considered a premalignant cancer that is localized to the organ of origin. As noted, it is reported separately and primarily relative to breast and skin cancer. Carcinoma in situ of the breast accounts for about 46,000 new cases every year, and in situ melanoma accounts for about 50,000 new cases annually.1 Cancer is the second leading cause of death in children between the ages of 1 and 14, with accidents remaining the most frequent cause of death in this age group. The most frequently occurring cancers in children are leukemia (primarily, acute lymphocytic leukemia), brain and other nervous system cancers, soft tissue sarcomas, non-Hodgkin lymphoma, and renal (Wilms’) tumor.1 Survival rates for childhood cancer have increased to 81% now because of improvements in treatment for many types of cancer over the past 2 decades. The result is an increasing population of long-term cancer survivors (or “overcomers” as some “survivors” prefer to call themselves). Currently, 1 in 900 young adults is a childhood cancer survivor. Long-term health problems related to the effects of cancer therapy are a major focus of this population group.4 It has been shown that, to varying degrees, long-term survivors of childhood cancer are at risk of developing second cancers and of experiencing organ dysfunction, such as cardiomyopathy, joint dysfunction, reduced growth and development, decreased fertility, and early death.5 The degree of risk of late effects may be influenced by various treatment-related factors such as the intensity, duration, and timing of therapy. Individual characteristics, such as the type of cancer diagnosis, the person’s sex, age at time of intervention, and genetic factors as indicated by, for example, family history of cancer, may also play a role in cancer recurrence and late effects of treatment.5,6 A new branch of medicine called preventive oncology has developed to address this important area. Preventive oncology or chemoprevention includes primary and secondary prevention. Chemoprevention is based on the hypothesis that certain nontoxic chemicals (e.g., retinoids, cyclooxygenase-2 [COX-2] inhibitors, hormonal agents) can be given preventatively to interrupt the biological processes involved in carcinogenesis and thus reduce its incidence. Currently, many clinical trials and studies have been devoted to the idea of chemical prevention of cancer development.7 Therapists can have an active role in both primary and secondary prevention through screening and education. Primary prevention involves stopping the processes that lead to the formation of cancer in the first place. According to the Guide to Physical Therapist Practice (the Guide),8 physical therapists are involved in primary prevention by “preventing a target condition in a susceptible or potentially susceptible population through such specific measures as general health promotion efforts.”8 Risk factor assessment and risk reduction fall under this category. Secondary prevention involves regular screening for early detection of cancer and the prevention of progression of known premalignant lesions such as skin and colon lesions. This does not prevent cancer but improves the outcome. The Guide outlines the physical therapist’s role in secondary prevention as “decreasing duration of illness, severity of disease, and number of sequelae through early diagnosis and prompt intervention.”8 Certain risk factors have been identified as linked to cancer in general (Table 13-1). More than half of all cancer deaths in the United States could be prevented if Americans adopted a healthier lifestyle and made better use of available screening tests.9 TABLE 13-1 HTLV-1, Human T-lymphotropic virus type 1; HIV, human immunodeficiency virus; UV, ultraviolet. According to the NCI, environmental factors, whether linked to lifestyle issues such as smoking and diet or exposure to carcinogens in the air and water, are thought to be linked to an estimated 80% to 90% of cancer cases.10 Some of the most common risk factors for cancer include the following: The majority of cancer incidence and mortality occurs in individuals aged 65 and older.11 With estimates from the U.S. Census Bureau predicting a rapid rise in the number of people 65 years old and older, the therapist must pay close attention to the client’s age, especially in correlation with a personal or family history of cancer. Many cancers, such as prostate, colon, ovarian, and some chronic leukemias, have increased incidence in older adults. The incidence of cancer doubles after 25 years of age and increases with every 5-year increase in age until the mid-80s, when cancer incidence and mortality reach a plateau and even decline slightly. Screening for age is discussed more completely in Chapter 2. Please refer to this section for information on screening for this red flag/risk factor. Racial/ethnic minorities account for a disproportionate number of newly diagnosed cancers. African Americans have a 10% higher incidence rate than whites and a 30% higher death rate from all cancers combined than whites.1,12 African Americans have the highest mortality and worst survival of any population, and diagnosis occurs at a later stage.1 The statistics have gotten worse over the past 20 years, and studies have shown that equal treatment yields equal outcomes among individuals with equal disease.13,14 Compared with the general population, African Americans die of cancer at a 40% greater rate (they are 30% more likely to die from heart disease). The Institute of Medicine (IOM) document, Unequal Treatment, Confronting Racial and Ethnic Disparities in Health Care, suggests that care providers may be part of the problem.14 Cancer statistics for Hispanic Americans compared with non-Hispanic Americans are becoming more available.1,15,16 There are lower rates of incidence and mortality from the four major cancer killers (breast, prostate, lung, colorectal) but a higher incidence and mortality from cancer with an infectious etiology (stomach, liver, uterine cervix, gallbladder). Therapists can offer health care education and cancer screening to this unique group of people. This will be increasingly common in our practice as our health care delivery system moves from an illness-based system to a health promotion–based system. For all groups, high-quality prevention and early detection and intervention can reduce cancer incidence and mortality.3 Screening for ethnicity is discussed more completely in Chapter 2. Please refer to this section for information on screening for this important red flag/risk factor. Hereditary cancer syndromes account for approximately 5% of breast, ovarian, and colon cancers. Both clients and providers are becoming aware of the potential therapeutic advantages of early identification of hereditary cancer risk.16a The small percentage of people who may be suspected of a hereditary cancer syndrome can be screened regarding personal and family medical history. Critical details, such as the cancer site and age at diagnosis, are needed for risk assessment. The following are some basic hallmarks of families who could have a hereditary cancer syndrome17: • Diagnosis of cancer in two or more relatives in a family • Diagnosis of cancer in a family member under the age of 50 • Occurrence of the same type of cancer in several members of a family • Occurrence of more than one type of cancer in one person • Occurrence of a rare type of cancer in one or more members of a family18 Obesity, diet, sedentary lifestyle, sexual practices, and the use of tobacco, alcohol, and/or other drugs make up the largest percentage of modifiable risk factors for cancer. Current data support the findings that obesity, inappropriate diet, and excess weight cause around one-third of all cancer deaths (Box 13-1).19,20 Increased body weight and obesity (as measured by body mass index [BMI], an approximation of body adiposity) are associated with increased death rates for all cancers and for cancers at specific sites, especially when combined with a sedentary lifestyle.21–25 Overweight and obesity may account for 20% of all cancer deaths in U.S. women and 14% in U.S. men.26 It is estimated that 90,000 cancer deaths could be prevented each year if Americans maintained a healthy body weight.21 Excess body weight increases amounts of circulating hormones, such as estrogens, androgens, and insulin,27,28 all of which are associated with cellular and tumor growth. It has also been shown that physical activity reduces the risk of breast and colon cancers and may reduce the risk of several other types of cancer by decreasing excess body weight and by actually decreasing the circulation of some of the growth-related hormones.29 In 1999, the American Institute for Cancer Research (AICR) estimated that at least 20% of all cancers could be prevented if everyone ate at least 5 ( Dietary guidelines were updated to 9 servings a day (equal to Specific factors associated with individual cancer types are known in some cases. For example, inadequate hydration is known to increase the risks of colon and bladder cancers. Alcohol consumption is linked with breast, head or neck, and gastrointestinal (GI) cancers. High dietary animal fat intake and tobacco use increase prostate cancer risk. Current smoking is an additive risk factor when combined with obesity for esophageal squamous cell carcinoma and lung and pancreatic cancers.33 Adenomatous polyps in the colon are known precursors of colorectal cancer. Sexually Transmitted Infections: Sexually transmitted diseases (STDs) or sexually transmitted infections (STIs) have been positively identified as a risk factor for cancer. Not all STIs are linked with cancer, but studies have confirmed that human papillomavirus (HPV) is the primary cause of cervical cancer (see Fig. 4-20). With current technology, high-risk HPV DNA can be detected in cervical specimens.34 HPV is the leading viral STI in the United States. More than 100 types of HPV have been identified; more than 30 types are transmitted sexually35: 23 infect the cervix and 13 types are associated with cancer (men and women). Infection with one of these viruses does not predict cancer, but the risk of cancer is increased. In 1970, 1 of every 300 Americans had an STI. Today, 3 million teenagers contract STIs every year; STIs affect about 1 in 4 teens who are sexually active and 50% to 75% of sexually active adults will develop HPV sometime in their lives.36 Nearly 50% of African-American teenagers have genital herpes.37 For every unwed adolescent who gets pregnant this year, 10 teenagers will get an STI.38,39 Tobacco Use: Tobacco and tobacco products are known carcinogens, not just for lung cancer but also for leukemia and cancers of the cervix, kidney, pancreas, stomach, bladder, esophagus, and oropharyngeal and laryngeal structures. This includes second-hand smoke, pipes, cigars, cigarettes, and chewing (smokeless) tobacco. Combining tobacco with caffeine and/or alcohol brings on additional problems. More people die from tobacco use than from use of alcohol and all the other addictive agents combined. In any physical therapy practice, clients should be screened for the use of tobacco products (see Fig. 2-2). Client education includes a review of the physiologic effects of tobacco (see Table 2-3). For a more complete discussion of screening for tobacco use, see Chapter 2. Occupation and Local Environment: Well-defined problems occur in people engaging in specific occupations, especially involving exposure to chemicals and gases. Exposure to carcinogens in the air and water and on our food sources may be linked to cancer. Isolated cases of excessive copy toner dust linked with lung cancer have been reported.40,41 Reactions can be delayed up to 30 years, making client history an extremely important tool in identifying potential risk factors. People may or may not even remember past exposures to chemicals or gases. Some may not be aware of childhood exposures. Taking a work or military history may be important (see Chapter 2 and Appendix B-14). In Utah, Nevada, and Arizona, the Radiation Exposure Compensation Act (RECA) was passed by Congress in 1990 after studies showed a possible link between hundreds of above-ground nuclear tests in the late 1950s and early 1960s and various cancers and primary organ diseases.42–45 Groundwater wells at old open-pit copper mines in various states have tested positive for uranium up to 40 times higher than legal limits. Hundreds of active wells tap into groundwater within 5 miles of these sites. Ionizing Radiation: Exposure to ionizing radiation is potentially harmful. Ionizing radiation is the result of electromagnetic waves entering the body and acting on neutral atoms or molecules with sufficient force to remove electrons, creating an ion. The most common sources of ionizing radiation exposure in humans are accidental environmental exposure and medical, therapeutic, or diagnostic irradiation. Some studies have reported the possibility of increased cancer risks, especially of leukemia and brain cancer, for electrical workers and others whose jobs require them to be around electrical equipment. Additional risk factors, however, such as exposure to cancer-initiating agents, may also be involved.46 Some researchers have looked at possible associations between electromagnetic exposure and breast cancer, miscarriages, depression, suicides, Alzheimer’s disease, and amyotrophic lateral sclerosis (ALS, or Lou Gehrig disease), but the general scientific consensus is that the evidence is not yet conclusive.46 Tanning lamps emit mostly UVA radiation with a few percentage content of UVB. Use of tanning lamps and beds can lead to significant exposure to UVA radiation. The risk of melanoma reportedly increases 75% when the use of tanning devices starts before age 30.47,48 The greater the frequency and intensity of exposure, the greater the risk. The risk is even higher for individuals using high-intensity or high-pressure devices.48 Despite known negative health effects from the use of indoor tanning devices, this practice is still very popular in the United States and Europe. Therapists have a role in client education, especially concerning reducing modifiable risk factors such as outdoor exposure to the sun without protection, exposure to sun lamps, and indoor tanning.49 Military Workers: Survivors of recent wars who have been exposed to chemical agents may be at risk for the development of soft tissue sarcoma, non-Hodgkin’s lymphoma, Hodgkin’s disease, respiratory and prostate cancers, skin diseases, and many more problems in themselves and their offspring. In early 2003, the military acknowledged that exposure to Agent Orange is associated with chronic lymphocytic leukemia among surviving veterans. There is also sufficient evidence of an association between Agent Orange and soft tissue sarcoma and non-Hodgkin’s lymphoma.50–52 The affected individual often presents with an unusual combination of multiorgan signs and symptoms. A medical diagnosis of chronic fatigue syndrome, fibromyalgia, or another more nonspecific disorder is a yellow flag. When and how to take the history and how to interpret the findings are discussed in Chapter 2. The mnemonic CH2OPD2 (Community, Home, Hobbies, Occupation, Personal habits, Diet, and Drugs) can be used as a tool to identify a client’s history of exposure to potentially toxic environmental contaminants.53 Each type of cancer has its own risk factors for cancer recurrence. For example, increased numbers of positive lymph nodes and negative estrogen/progesterone receptor (ER/PGR) status for breast cancer survivors are risk factors (Case Example 13-1). A positive ER/PGR status lowers the risk of breast cancer recurrence because it allows the woman to receive treatment for prevention of recurrence according to age and stage of cancer. Cancer prevention begins with risk factor assessment and risk reduction. The key to cancer prevention lies in minimizing as many of the individual modifiable risk factors as possible. The AICR estimates that recommended diets, together with maintenance of physical activity and appropriate body mass, can in time reduce cancer incidence by 30% to 40%. At current rates, on a global basis, this represents 3 to 4 million cases of cancer per year that could be prevented by dietary and associated means.20 There are some simple steps to take in starting this process. The first is to assess personal/family health history. Note any cancers present in first-generation family members. Some helpful tools are available for assessing cancer risk. The Harvard School of Public Health offers an interactive tool to estimate an individual’s risk of cancer and offers cancer prevention strategies. Anyone can benefit from it, but accuracy is greatest for adults over age 40 who have never had any type of cancer. It is available at the Harvard Center for Cancer Prevention website at www.yourcancerrisk.harvard.edu. For example, 90% of colon cancer cases and deaths can be prevented. The ACS provides a summary of risk factors and early detection screening tests for many types of cancer, including colon cancer. (This information is available at www.cancer.org/Healthy/InformationforHealthCareProfessionals/ColonMDClinicansInformationSource/index, on the ACS website.) The Gail Model Risk Assessment Tool can also be used to assess personal risk for breast cancer. It is available at www.halls.md/breast/riskcom.htm (Breast Cancer Risk [Gail Model] Calculation Methods). With the advent of the Genome Project, the sequencing of all genes in humans is nearly completed. Along with this discovery has come the development of a new biology of genetics called genomics. Understanding gene–environment interaction will be a major focus of genomics-based public health.54 Toxicogenomics, the development of molecular signatures for the effects of specific hazardous chemical agents, will bring to our understanding ways to track multiple sources of the same agent, multiple media and pathways of exposures, multiple effects or risks from the same agent, and multiple agents that cause similar effects.54 Defects may occur in one or more genes. Damage may occur in genes that involve the metabolism of carcinogens or in genes that deal with the DNA-repair process.55 An important discovery in the area of gene identification related to cancer suppression is discovery of the p53 tumor-suppressor gene.56 The p53 gene encodes a protein with cancer-inhibiting properties. Loss of p53 activity predisposes cells to become unstable and more likely to take on mutations. Mutation of the p53 gene is the most common genetic alteration in human cancers.57 It is possible that genetic defects combined with lifestyle or environmental factors may contribute to the development of cancer. For example, there is a known increase in risk of breast cancer in American women born after 1940 who have BRCA1 or BRCA2 mutation. This suggests that changes in the environment or lifestyle increased the risk already conferred by these genes.58 Air pollution is moderately associated with increased lung cancer, but when combined with exposure to tobacco smoke, the risk increases dramatically. About 50% of all people lack the GSTM1 metabolic gene that can detoxify tobacco smoke and air pollution. People who have this genetic defect and who have heavy exposure to pollution may have a higher risk of developing lung cancer.55 Tissues affected include connective tissue such as bone and cartilage (discussed subsequently under Bone Tumors), muscle, fibrous tissue, fat, and synovium. The different types of sarcomas are named for the specific tissues affected (e.g., fibrosarcomas are tumors of the fibrous connective tissue; osteosarcomas are tumors of the bone; and chondrosarcomas are tumors arising in cartilage) (Table 13-2). TABLE 13-2 Classification of Soft Tissue and Bone Tumors Data from Purtilo DT, Purtilo RB: A survey of human disease, ed 2, Boston, 1989, Little, Brown; Phipps W, et al: Medical-surgical nursing: concepts and clinical practice, ed 4, St. Louis, 1990, Mosby. As a general category, sarcoma differs from carcinoma in the origin of cells composing the tumor (Table 13-3). As mentioned, sarcomas arise in connective tissue (embryologic mesoderm), whereas carcinomas arise in epithelial tissue (embryologic ectoderm) (i.e., cellular structures covering or lining surfaces of body cavities, small vessels, or visceral organs). Although there are many websites related to cancer, we recommend what the physicians use: the well-known and respected National Comprehensive Cancer Network (NCCN). The NCCN Clinical Practice Guidelines in Oncology are recognized by clinicians around the world as the standard for oncology care. The NCCN now has consumer versions of its clinical practice guidelines (www.nccn.com). Other reliable sites include Abramson Cancer Center of the University of Pennsylvania at www.oncolink.org or www.oncolink.com and the National Cancer Institute (www.cancer.gov/). For self-assessment of cancer (and other diseases), go to www.yourdiseaserisk.wustl.edu. A distal or distant metastasis is the distant arrest, growth, and development of a malignant lesion to another organ (e.g., lung, liver, brain). Within the categories of invasive and metastatic tumors, four large subcategories of malignancy have been identified and classified according to the cell type of origin (see Table 13-3). Metastatic spread can occur as late as 15 to 20 years after initial diagnosis and medical intervention. As many as 70% of people who die of cancer have been shown on autopsy to have spinal metastases; up to 14% exhibit clinical symptomatic disease before death.59 For these reasons, the therapist must take care to conduct a screening interview during the examination, including past medical history of cancer or cancer treatment (e.g., chemotherapy, radiation). Use the personal/family history form (see Fig. 2-2) in Chapter 2 to assess for a personal or first-degree family history of cancer. When asked about a past medical history of cancer, clients may say “No,” even in the presence of a personal history of cancer. This is especially common in those clients who have reached and/or passed the 5-year survival mark. Always link these two questions together: Cancer cells can spread throughout the body through the bloodstream (hematogenous or vascular dissemination), via the lymphatic system, or by direct extension into neighboring tissue or body cavities (Fig. 13-2). Once a primary tumor is initiated and starts to move by local invasion, tumor angiogenesis occurs (blood vessels from surrounding tissue grow into the solid tumor). Tumor cells then invade host blood vessels and are discharged into the venous drainage. The relatively high incidence of anatomic skip metastasis can be attributed to aberrant distribution of lymph nodes.60 Multiple interconnections between the lymphatic and hematogenous systems may also allow transport of tumor cells via the arterial or venous blood supply, bypassing some lymph nodes while reaching other more distant nodes.61 Primary bone cancer, such as osteogenic sarcoma, initially metastasizes via blood to the lungs. Prostate cancer spreads via lymphatics to pelvic and vertebral bones, sometimes appearing as low back and/or pelvic pain radiating down the leg. The more common cancers and their metastatic pathways are provided in Table 13-4. The high proportion of bone metastases in breast, prostate, and lung cancers is an example of selective movement of tumor cells to a specific organ. For example, in breast cancer, it is thought that the continuous remodeling of bone by osteoclasts and osteoblasts predisposes bone to metastatic lesions.62,63 For some cancers, such as malignant melanoma, no typical pattern exists, and metastases may occur anywhere. Increased tumor contact with the circulatory system provides tumors with a mechanism to enter the general circulation and colonize at distant sites. Both vascular endothelial growth factor (VEGF) and fibroblast growth factor stimulate proliferation of vascular cells and even allow the newly formed blood vessels to be easily invaded by the cancer cells that are closely adjacent to them.64 Resection of tumors without clear margins has the potential to provide remaining tumor cells with a means of metastasizing as new blood vessels form during the healing process. Mechanical transport rather than metastasis may be another mechanism of cancer spread. Two potential modes of benign mechanical transport (BMT) have been detected: lymphatic transport of epithelial cells displaced by biopsy of the primary tumor and breast massage–assisted sentinel lymph node (SLN) localization.65 Samples of malignant tissues must be very carefully excised by surgeons who are expert in the biopsy of malignant tissues.66,67 The risk of local recurrence is increased when intralesional curettage alone is performed.68 Recurrence along the surgical pathway has been reported for some tumors following needle biopsy.69,70 It is hypothesized that this recurrence is the result of intraoperative seeding. Poorly planned biopsies or incomplete tumor resection increases the risk of local recurrence and metastasis. The biopsy tract should be excised when complete tumor removal occurs.71 A second mode of BMT may be the pre-SLN breast massage used to facilitate the localization of SLNs during breast cancer staging. Mechanical transport of epithelial cells to SLNs has been verified. The significance of small epithelial clusters in SLN is unknown; further research is needed before changes are recommended for biopsy and SLN-localizing practices.65 Each of these systems has a core group of most commonly observed signs and symptoms that will be discussed throughout this section (Table 13-5). TABLE 13-5 Signs and Symptoms of Metastases* CNS, Central nervous system; ABCDE, asymmetry, border, color, diameter, evolving.
Screening for Cancer
Cancer Statistics
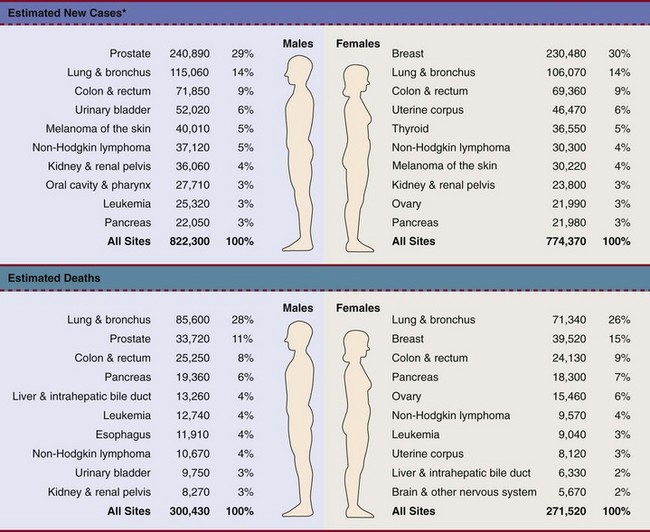
*Estimates are rounded to the nearest 10 and exclude basal and squamous cell skin cancers and in situ carcinoma except urinary bladder. (From Siegel R, Ward E, Brawley O, et al: Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths, CA Cancer J Clin 61:212-236, 2011. © 2011 American Cancer Society.)
Childhood Cancers
Risk Factor Assessment
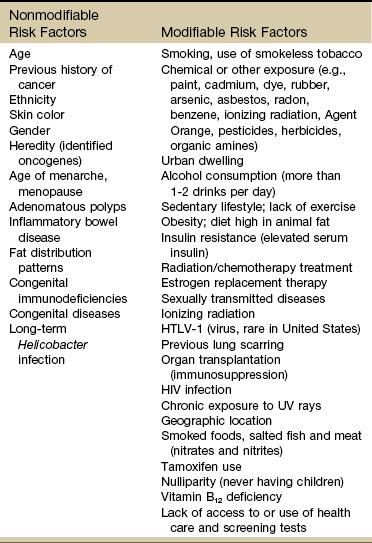
Known Risk Factors for Cancer
Age
Ethnicity
Family History and Genetics
Environment and Lifestyle Factors
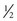 cup) servings of fruits and vegetables each day.29 Numerous resources on nutrition and its influence in preventing and treating cancer are available.30–32
cup) servings of fruits and vegetables each day.29 Numerous resources on nutrition and its influence in preventing and treating cancer are available.30–32
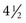 cups) for overall health and chemoprevention in January 2005 by the U.S. Department of Health and Human Services (HHS) in the publication Dietary Guidelines for Americans 2005. The Guidelines provide authoritative advice for people 2 years of age and older about how good dietary habits can promote health and reduce risks of major chronic diseases. The numbers of avoidable cancers through avoidance of excess weight are substantial.33
cups) for overall health and chemoprevention in January 2005 by the U.S. Department of Health and Human Services (HHS) in the publication Dietary Guidelines for Americans 2005. The Guidelines provide authoritative advice for people 2 years of age and older about how good dietary habits can promote health and reduce risks of major chronic diseases. The numbers of avoidable cancers through avoidance of excess weight are substantial.33
Risk Factors for Cancer Recurrence
Cancer Prevention
Genomics and Cancer Prevention
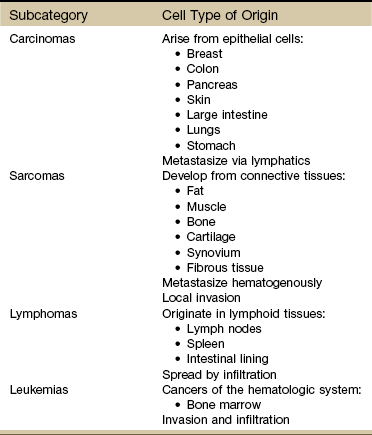
Major Types of Cancer
Resources
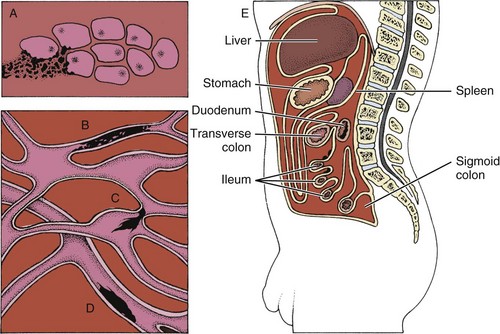
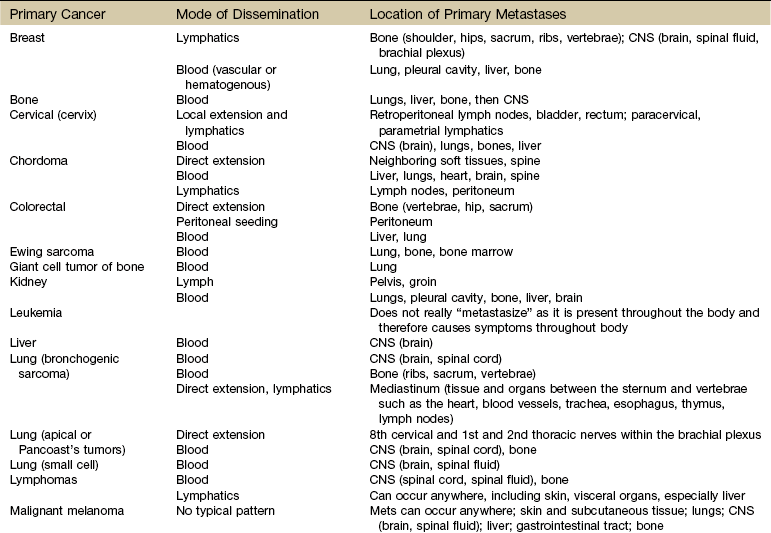
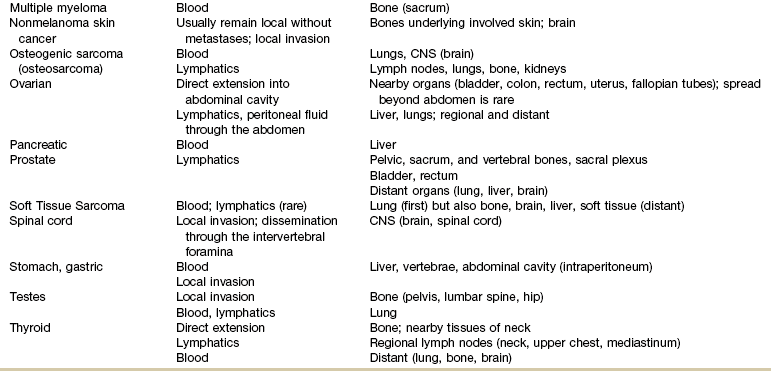
Metastases
Mechanisms and Modes of Metastases
Benign Mechanical Transport
Clinical Manifestations of Malignancy
Screening for Cancer