Sacrectomy
Peter S. Rose
Franklin H. Sim
Michael Yaszemski
Curative treatment of most primary sacral malignancies requires en bloc sacrectomy. Surgery in this area is challenging due to the complexity of the pelvic anatomy, adjacent visceral and vascular structures, and frequent compromise of spinopelvic continuity.
INDICATIONS
The most common indication for sacrectomy is primary sacral malignancy requiring resection for cure.
More rarely, sacrectomy is indicated for patients with primary or recurrent pelvic visceral tumors (most commonly colorectal carcinoma with sacral involvement by direct extension) and no evidence of metastatic or nodal disease.
The techniques described may be adapted to intralesional treatment of benign tumors (e.g., osteoblastoma, aneurysmal bone cyst) in a less aggressive fashion.
Select benign aggressive sacral tumors may be considered for en bloc resection, particularly if small or recurrent.
CONTRAINDICATIONS
The presence of disseminated malignancy is a strong relative contraindication for sacrectomy. The procedure is of such magnitude and generally entails deliberate neurologic defects with frequent loss of bowel, bladder, sexual, and potentially lower extremity function that it is usually inappropriate to pursue without curative intent.
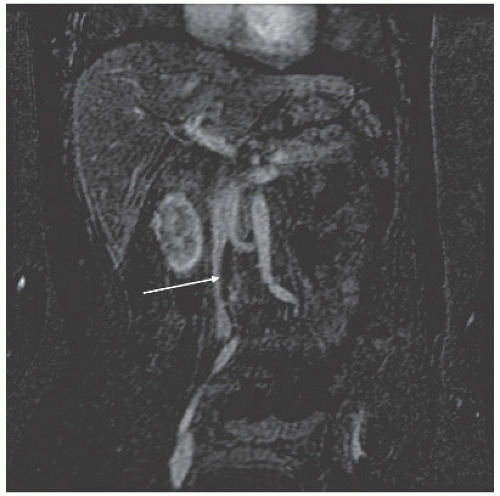
We have noted that patients with tumor thrombus in the iliac veins or vena cava by sarcoma predictably have a rapid development of metastatic disease and demise. The suggestion of this on preoperative imaging prompts catheter-directed biopsy at our institution, and its finding at time of surgery prompts abortion of resection (Fig. 7.1).
The inability to obtain a tumor-free margin of resection is similarly a relative contraindication. However, we are aggressive in pursuing this with frequent en bloc resections encompassing the pelvis, lumbar spine, rectum, urogenital, and vascular structures to obtain en bloc tumor resection.
The medical fitness of the patient for surgery also frequently enters into the calculation of surgery. Patients receiving chemotherapy frequently require alterations in their chemotherapy schedules to allow for surgery of this magnitude. All patients are subject to an intense preoperative medical evaluation including a dobutamine stress echocardiogram for (a) anyone with known cardiovascular disease; (b) men over age 40; or (c) women over age 50.
PREOPERATIVE PLANNING/GENERAL CONSIDERATIONS
Staging and accurate diagnosis are key in the evaluation of patients with sacral malignancy. Systemic staging includes CT of the chest, abdomen, and pelvis and bone scan for all patients; the role of PET-CT in staging is currently evolving. Local tumor imaging is best provided by MR scan. All tumors are biopsied prior to surgery by CT-guided needle biopsy in or near the midline to facilitate biopsy tract removal.
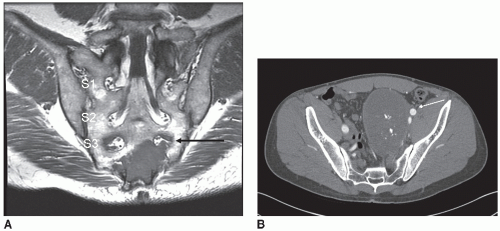
Tumor imaging is accomplished using contrast-enhanced MR. Sagittal images and coronal oblique images (coronal images in the plane of the sacrum) are key to localizing tumor extent and adjacent structure involvement. Selected patients benefit from CT angiography to evaluate the pelvic vasculature (Fig. 7.2).
Resections at or below the level of the S2 neuroforamen are generally resected through a posterior approach unless there is involvement of pelvic visceral or vascular structures. Given the need to obtain an oncologic mar gin, this generally implies lesions at or below the S2/3 vestigial disc.
Lesions cephalad to this or involving pelvic structures are treated first with anterior mobilization of pelvic structures, vessel ligation, and unicortical anterior sacral osteotomy. It is our practice to harvest a pedicled myocutaneous rectus abdominus flap and tuck it into the abdomen with the anterior procedure. Tumor resection
is then completed through a posterior approach, and the rectus flap is pulled through the abdomen and rotated to assist in wound closure and reconstruction of the posterior abdominal wall. Unless the rectum is devascularized and requires resection with the tumor specimen, we separate the anterior and posterior stages by 48 hours.
is then completed through a posterior approach, and the rectus flap is pulled through the abdomen and rotated to assist in wound closure and reconstruction of the posterior abdominal wall. Unless the rectum is devascularized and requires resection with the tumor specimen, we separate the anterior and posterior stages by 48 hours.
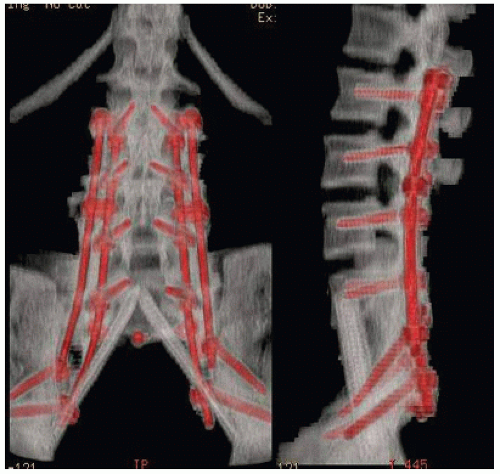
Resections cephalad to the S1 neuroforamen require spinopelvic reconstruction. Our preferred method involves a “cathedral” technique using fibula autografts or allografts and posterior spinal instrumentation to be described below (Fig. 7.3). Allograft fibulae are used unless the patient has had prior radiotherapy, in which case consideration is given to vascularized fibula transfer.
Biopsy tracts are excised in continuity with the surgical specimen. In the case of posterior-only resections, gluteal V-Y advancement flaps are commonly used to facilitate wound closure. Ureteral stents are placed preoperatively in all patients undergoing anterior procedures. Patients are at risk of venous thromboembolic disease but also at risk of significant perioperative bleeding. In patients undergoing dorsal-only resections, we place a removable Inferior Vena Cava filter preoperatively. If anterior and posterior procedures are needed, the IVC filter is placed between the anterior and posterior stages so as not to interfere with vena cava mobilization.
ANTERIOR TECHNIQUE—RESECTIONS ABOVE S2 OR INVOLVING PELVIC VISCERA
Positioning and Landmarks
The patient is positioned supine on a regular operating table. Critical landmarks for anterior exposure are the sacral promontory, the anterior sacral foramina, the sacroiliac joints, and the sciatic notches. Resections requiring anterior exposure are usually performed through the S1 segment or higher, so the sacral promontory provides adequate localization. Large tumors frequently require inclusion of the medial iliac bones with the specimens to predictably remove the sacroiliac joints without violation. These joints can be palpated and osteotomies begun at the sciatic notches and brought cephalad.
Surgical Technique
Bilateral ureteral stents are placed to facilitate ureteral identification. A midline laparotomy incision is made with planned harvest of a vertical rectus abdominus myocutaneous flap. The timing of flap harvest is at the discretion of the plastic surgeon. It is most efficiently harvested at the conclusion of the anterior procedure to minimize risk of pedicle avulsion during the case. In rare cases, a transverse rectus abdominus flap is raised when a very large posterior soft tissue defect is expected (the TRAM flap has greater bulk).
The abdomen is approached by transperitoneal approach in most cases (in cases of hemisacrectomy, a retroperitoneal approach may be used). The rectum is mobilized or transected as indicated by tumor extent. The pelvic vasculature is identified. If thrombus is palpated, veinotomy is made and the specimen analyzed. If tumor is found, the procedure is aborted; if bland, thrombectomy is performed and the procedure continues. The middle sacral vein and the internal iliac artery and vein (and all accessible branches) are ligated. If necessary, a plane between the tumor and the pelvic sidewall is developed. The location of sacral and/or pelvic osteotomies is localized using anatomic landmarks and lateral radiography as necessary. The L5/S1 disk is a reliable landmark, and sacral neuroforamina can be counted down from this.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree