Rotator Cuff Repair Part I. Arthroscopic Approach
Carlos A. Guanche MD
History of the Technique
The treatment of rotator cuff tears has certainly improved over the past few years as a result of an increased understanding of the mechanics and anatomy of the involved tissues. However, the quantum leap has occurred as a result of the increasing use of the arthroscope in the management of these injuries. Initially, the use of the tool was confined to the diagnosis and occasionally management of subacromial bone spurs. The thinking has now shifted to an all-arthroscopic treatment. This is reminiscent of the history of the treatment of meniscal tears, chronic anterior cruciate ligament (ACL) insufficiency, shoulder impingement, and shoulder instability. Over time, clinical practice favors the arthroscopic treatment despite initial skepticism and lack of scientific evidence to support the procedures.
All-arthroscopic cuff repair is probably the most technically challenging of the commonly performed procedures done in the shoulder. It constitutes a natural extension of the arthroscopic-assisted mini-open repair. The latter has many of the advantages of an all-arthroscopic repair with less of the technical demands pertaining to the mobilization and securing of the rotator cuff to the greater tuberosity. No matter which repair technique is chosen, some fundamental principles of cuff repair should be applied.1 These include preservation (or meticulous repair) of the deltoid, adequate subacromial decompression, surgical release to produce a freely mobile muscle-tendon unit, secure fixation of the tendon to the greater tuberosity, and closely supervised rehabilitation.
There clearly is no best technique. The most important factor is the individual surgeon’s experience and comfort level. A good open repair will always be superior to a badly performed arthroscopic one. There are also technical considerations related to size of tear, quality of tissue, available equipment, and patient expectations. Preoperative imaging can assist with planning and discussion of options with the patient. A surgeon wishing to make the transition to arthroscopic repair should do so in a stepwise and careful fashion, first perfecting arthroscopic acromioplasty and the mini-open technique followed by progression to all-arthroscopic techniques.
The history of arthroscopic rotator cuff repairs can be traced back to the pioneers of shoulder arthroscopy such as Lanny Johnson who began repairing rotator cuff tears with a removable staple. Following that, Eugene Wolf pioneered the use of suture anchors in the subacromial space. This was closely followed by Steven Snyder,2 who has written and presented extensively on the suture anchor technique dating back to the 1980s. More recently, Stephen Burkhart3 has further advanced the surgery with the use of a variety of tools designed to expedite the surgical procedure.
Indications and Contraindications
There are two common reasons for surgical intervention in rotator cuff tears. They are pain and weakness. The two are commonly inseparable when it comes to the treatment of the entity. In general, as has been discussed above, the type of procedure should not matter as much as the ability to provide an adequate repair by whatever means are used. However, the use of minimally invasive procedures clearly impacts the patient’s decision on whether or not to proceed with the intervention.1
Historically, open repair has been espoused as reproducibly improving strength as well as reducing pain. Recently, the ability to reproducibly improve function has also been shown with arthroscopic techniques. The outcomes appear to be as good with open, mini-open, or arthroscopic
techniques, the only caveat being that the surgeon must be proficient in the chosen technique.4
techniques, the only caveat being that the surgeon must be proficient in the chosen technique.4
The indication for repair of a tear (either complete or partial) is that it remains symptomatic despite a supervised course of physical therapy with or without the judicious use of subacromial cortisone injection(s). Although complete tears have historically been the only types to undergo repair, there is a growing body of knowledge that appears to support the repair of partial thickness tears in higher-level throwing athletes and laborers that continue to be symptomatic despite conservative measures. Initially, arthroscopic repair was described for tears measuring less than 2 cm. With the growing armamentarium of surgical tools and techniques, such as margin convergence, side-to-side repair, and transtendon repair, the size of the tear has become irrelevant to many surgeons.2,3
In addition to the above considerations, it is important to acknowledge that there are typically several other areas that need to be addressed at the time of rotator cuff repair. These most commonly include the need to perform a subacromial decompression (and possibly a distal clavicle resection), but often also the management of labral and biceps pathologies. The critical factor in this consideration is the time available for a successful arthroscopic procedure. The allotted time for surgical repair of the cuff is limited by fluid extravasation. Typically, the surgeon must accomplish any associated procedures in a minimal amount of time in order to accomplish the more daunting task of rotator cuff repair before fluid extravasation precludes successful repair.
Contraindications to arthroscopic repair include all of the usual parameters commonly employed in open and mini-open techniques. These include the attempt to repair obviously degenerative cuff tears showing not only intraoperative degenerative changes but also fatty infiltration and muscle atrophy on imaging studies. In addition, the quality of the bony bed available for the repair must be such that anchors can be successfully implanted and maintain their purchase. Finally, as discussed above, the techniques of all-arthroscopic repair can be difficult in inexperienced hands. The physician should be proficient with the techniques and should also be ready to convert to either an open or mini-open technique should the complexity of the procedure preclude a good outcome.
Surgical Technique
The options for positioning during arthroscopic rotator cuff repair include the beach chair and lateral positions. The literature is replete with good outcomes in either position. This chapter will assume that the patient is in the lateral decubitus position with traction applied across the upper extremity in standard fashion. The basic setup for lateral decubitus arthroscopic surgical intervention in the shoulder has been well described by multiple authors and will not be discussed in detail here.5
The procedure can be performed under either general or regional anesthesia, depending on the experience of both the surgeon and the anesthetist involved in the procedure. The use of hypotensive anesthesia is helpful in most cases, especially with surgeons who are beginning the technique. This is obviously accomplished with a general anesthetic. The systolic pressure should be ideally maintained at 90 mm Hg. In addition, the use of preemptive analgesia is highly desirable. Typically, prior to sterile prep and drape of the patient, the subacromial space is infiltrated with 30 cc of 0.5% Marcaine with 1:100,000 epinephrine. This allows for both postoperative comfort for the patient and also simplifies anesthetic management.6 An interscalene block is also effective for intraoperative and postoperative pain control and allows for easier blood pressure control intraoperatively.
Standard anterior and posterior glenohumeral portals are established with the arthroscope being inserted in the posterior portal. The procedure begins with the intra-articular visualization of the entire glenohumeral joint and management of any additional pathology that may be present. The articular surfaces of the glenoid and humerus should be carefully evaluated for concomitant damage. Similarly, the labral structures should be assessed and any pathology that is found should be addressed first before turning to the rotator cuff.
Following completion of any work in the glenohumeral joint, the rotator cuff is now addressed. The important factors of size of the tear (in the anterior to posterior and medial to lateral directions) should be delineated. In addition, full-thickness tears must be assessed for the pattern of tearing.7 This is important to decide the order of repair, including considerations for side-to-side suturing (“margin convergence”), prior to suture anchor implantation. In addition, whether or not the tear is repairable must also be addressed. The factors employed in this consideration include whether the tear is immediately repairable or if significant soft tissue releases must be undertaken. This is, perhaps, the critical junction in the decision-making process for most surgeons. If extensive mobilization and complex suturing patterns will be required, then the surgeon must decide whether these techniques fit within their proficiency. If there is any doubt, then the arthroscopic procedure should be abandoned and traditional techniques employed.
Partial thickness tears are increasingly documented and newer techniques allow transtendon repair. The partial thickness tear may be equally symptomatic to a complete tear in those with excessive physical demands. The average thickness of a rotator cuff insertion is approximately 15 mm.8 It is, therefore, important to document the thickness of tearing involved in any area. Once this is documented, the patient’s size, activity level, and degree of clinical weakness are taken into account in order to decide whether to debride or repair a partial tear. The traditional indication for repair of a partial thickness cuff tear has been one greater than 50% of the thickness of the tendon. However, this is weighed against the patient’s age, activity level, and physical demands.
A 30% tear in an elderly and relative sedentary individual is clearly different than the same tear in a professional baseball player. The general recommendation is that a greater than 50% partial tear should be repaired either with a transtendon technique or by completing the tear and repairing it through whatever means the surgeon decides. Those tears involving less than 50% of the thickness should be treated with debridement of the torn portion as well as consideration of other anatomic factors, including labral and subacromial pathology.
A 30% tear in an elderly and relative sedentary individual is clearly different than the same tear in a professional baseball player. The general recommendation is that a greater than 50% partial tear should be repaired either with a transtendon technique or by completing the tear and repairing it through whatever means the surgeon decides. Those tears involving less than 50% of the thickness should be treated with debridement of the torn portion as well as consideration of other anatomic factors, including labral and subacromial pathology.
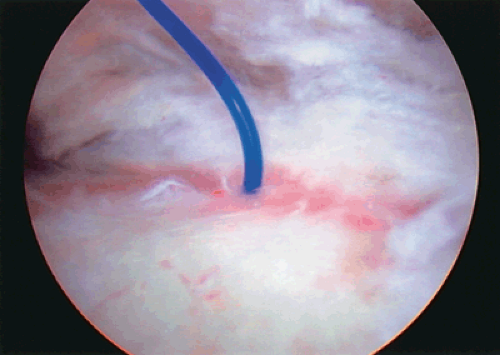
In cases where there is a clear intra-articular partial tear, yet the depth is not completely known, it is useful to insert a suture through the area of partial tearing as a marker suture.2 This is accomplished with the use of a spinal needle that is placed near the anterolateral corner of the acromion through the area of partial tearing and visualized in the joint. Once this is visualized, a large diameter suture is placed through the needle and visualized in the joint. Several centimeters of suture are advanced into the joint and the needle is withdrawn. The suture is now a useful landmark to visualize the subacromial portion of the partial inner surface tear (Fig. 9-1). Any tearing visualized in this portion of the cuff should then be taken into account when deciding on the overall size of the partial tear.
Once the intra-articular visualization is completed, the subacromial space is entered through the posterior portal. The arthroscopic sheath is placed immediately adjacent to the acromion and swept in a medial to lateral direction in order to release any subacromial adhesions that may be present. Palpating the coracoacromial ligament with the trocar delineates the most anterior portion of the subacromial space. Once oriented, the trocar is withdrawn and the arthroscope is placed in the sheath. A lateral portal is now established using direct visualization. In general, the ideal location for the portal is about 2 cm below the lateral acromion at about the midportion of the bone. The most common error in placing the portal is superior placement. This becomes a critical factor once it is necessary to reach further medially with grasping tools and suture passers. In general, it is much better to err on the side of being too low rather than too high since this will not preclude access to the medial structures.
The use of arthroscopic cannulas in all of the portals is encouraged for entering the subacromial space, thus limiting the trauma to the soft tissues and increasing distension of the space. The cannulas that are employed are 7-mm clear devices (Crystal Cannula; Arthrex, Inc, Naples, Fla) that allow visualization of instruments and sutures within the sheath of the device as they are advanced. These are maintained throughout the rest of the procedure. If the arthroscope is placed in a particular portal, it is simply inserted through the cannula in many cases.
Once the lateral portal is established, a shaver is employed to perform a thorough bursectomy. In addition, it is useful to have a cautery device available for control of any bleeding that may arise during the debridement. This may be either a standard electrocautery instrument or a newer bipolar or monopolar radiofrequency device. It is safe to start debriding white tissue laterally and avoiding yellowish tissue medially. The yellowish, fatty tissue is well vascularized and can lead to bleeding. Regardless of the method chosen, it is important to perform a complete bursectomy at the beginning of the procedure. Not only does this allow for complete visualization of the subacromial space, but it also prevents impaired visualization later in the procedure when fluid is absorbed by many of the periarticular structures.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree